Abstract
The perceived threat to public health from dental unit water line (DUWL) contamination comes from opportunistic and respiratory pathogens such as
From Volume 44, Issue 4, April 2017 | Pages 284-292
The perceived threat to public health from dental unit water line (DUWL) contamination comes from opportunistic and respiratory pathogens such as

It was first recognized in the 1960s that water sampled from the DUWL contained large numbers of organisms in the range 104–106 colony forming units (cfu)/mL.1
Two decades later the American Dental Association (ADA) set the goal of <200 cfu/mL of aerobic heterotrophs as the standard for dental unit water lines.2 This figure is reflected in the current recommendation for England, Wales and Northern Ireland for dental unit water quality of 100–200 cfu/mL of aerobic heterotrophs at 22°C.3 Since then, our understanding of the physiology of the biofilm,4 incidence data on legionellae in dental practice5,6 and the first proven case of Legionella transmission and death of a patient associated with contaminated DUWLs have transformed the management of DUWLs.7 Challenges caused by Legionella contamination have not just affected dentistry but, around the world, there have been major outbreaks of Legionnaires' disease with resultant deaths of many of those infected.8 Indeed, dentistry is specifically included in the Approved Code of Practice for Legionnaires' disease control in water systems, which states that DUWL management is viewed in the context of the dental practice's overall hot and cold water supply systems.9
The effluent organisms from DUWLs essentially originate from the biofilms on the luminal surface of the water line. Biofilms are defined as an organized community of microbes attached to a biotic or an abiotic surface, and encased in an extracellular polymeric substance.
The usual source of feed water to the dental unit which nourishes the luminal biofilm on DUWL is from the potable municipal supply or, preferably, from an independent bottled water supply.3 After entering the unit proper, the water passes through a multichannel control box which distributes the water to dental unit water lines (hoses) feeding the high-speed handpiece, the air/water syringe and the ultrasonic scaler. DUWLs have a number of characteristics that promote biofilm growth unless they are regularly cleaned and disinfected. For instance, they have a very small bore but a high surface area, slow flow rate and the whole column of water in the DUWL can be stagnant for long periods of time. Biofilms develop rapidly in such systems within about 8 hours,6 forming a mature bacterial community enveloped in a protective matrix within 6 days that is capable of shedding 104–106 cfu/ml if left untreated.4
Biofilms on the lumen walls of DUWLs act as a reservoir of micro-organisms. A wide range of species of environmental and oral bacteria, fungi, amoebae and protozoa have been isolated from DUWLs. The majority are harmless, so called saprophytic, Gram negative environmental bacterial species. However, the lipopolysaccharide (LPS) cell wall of all Gram-negative bacteria, a potent source of non-infectious endotoxins, can evoke mild or severe inflammatory responses leading to diseases such as periodontitis and systemic, septic shock.
A high bacterial load in the DUWL equates with a high endotoxin concentration. Endotoxin has been detected in dental unit water up to 50010–2,56011 Endotoxin Units/mL compared to a mean level of 66 EU/mL found in water samples collected from adjacent sinks. Putnins et al11 have suggested that the endotoxin in DUWLs might stimulate proinflammatory cytokines in gingival tissue during surgery and adversely affect healing. Endotoxins are known to trigger occupational asthma in susceptible people. An association between the onset of occupational asthma in dentists and the concentration of bacteria in their DUWLs has been demonstrated in a cross-sectional multicentre DUWL survey of 265 dentists in general dental practice. Fourteen percent of the dentists enrolled in the study reported ‘ever having suffered’ from asthma either in childhood or as an adult.12 Endotoxin derived from DUWL bacteria may account for this increase in occupational asthma, as dentists who were exposed in their dental surgery to total aerobic counts at 37°C at a concentration of 200 cfu/mL were more likely to report symptoms of asthma ‘since starting dentistry’.12
Legionellae can cause a variety of respiratory infections (Figure1). Legionnaires' disease, or Legionellosis, is a severe infection which typically presents as pneumonia and symptoms may include a high fever, chills, cough, muscle aches, headaches, and diarrhoea.13 Although healthy young adults can develop Legionnaires' disease in two thirds of patients, there are other co-morbidities and risk factors.13 Legionnaires' disease is a notifiable disease in England and Wales and is a relatively uncommon cause of pneumonia, with approximately 350–500 cases per annum in the UK, the majority of which are due to Legionella pneumophila serogroup 1.13 European Legionnaires' Disease Surveillance Network in 2016 reported the notification rate of Legionnaires' disease in the EU/EEA in 2014 was 13.5 cases per million/population, the highest ever observed, and suggested that this may be due to favourable meteorological conditions that supported the growth of legionellae.14. Although, when viewed from a global burden of disease perspective, the incidence of Legionellosis is very low, compared to other major respiratory infectious diseases such as tuberculosis, which has an estimated incidence of 10.5 per 100,000 of the population (5,758 cases) in 2015 in the UK,15 and an estimated 10.4 million TB cases worldwide.16 However, regardless of the relatively small number of cases of Legionellosis, registered providers of dental services have a duty of care to prevent and control the risks from contamination of practices' water supplies with legionellae. The registered provider of dental services is legally obliged to take account and comply with the standards as specified within ACOP L89 for water quality standards in healthcare premises.
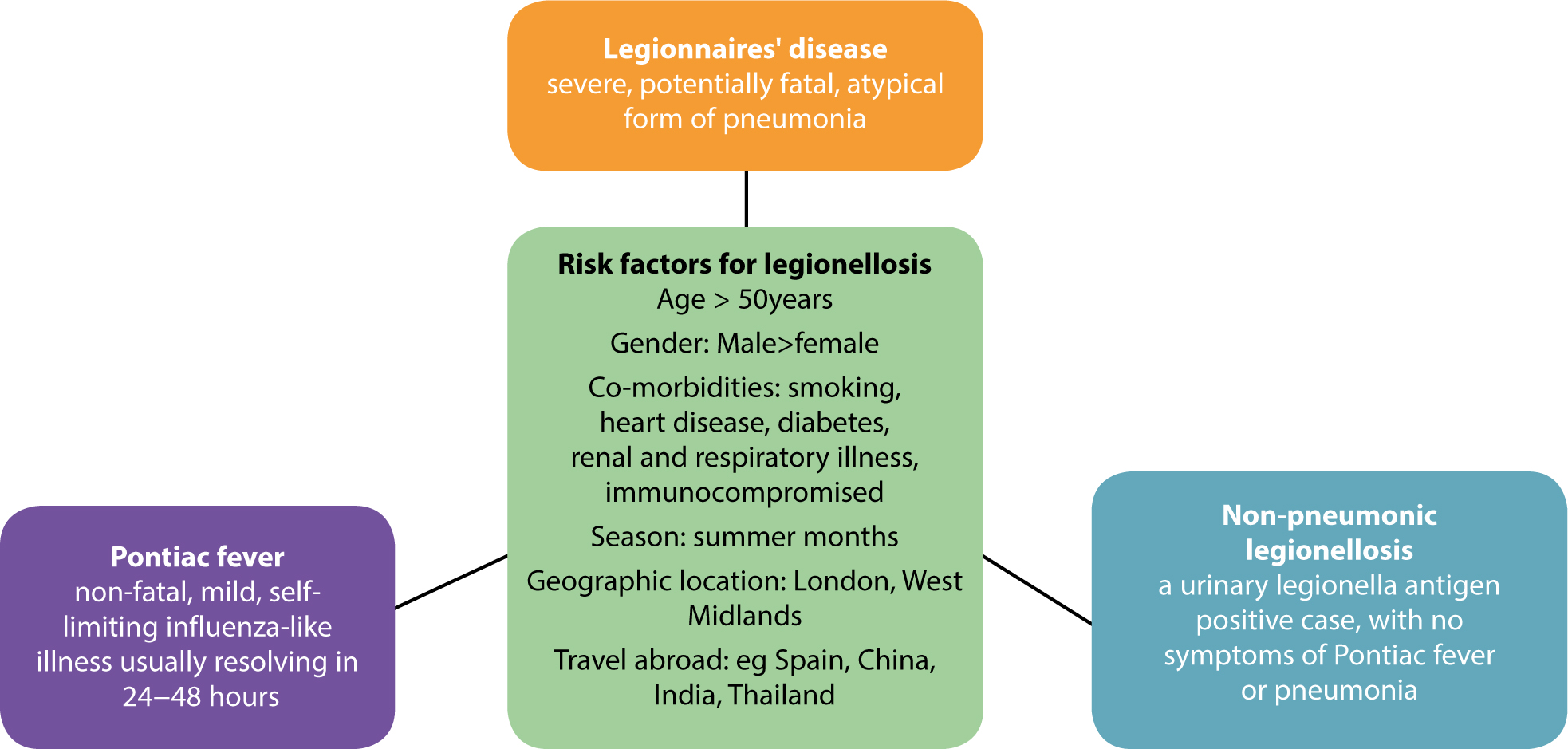
There is no reported person to person transmission of legionellae, and infection is essentially associated with exposure to a contaminated water source. Legionellae are ubiquitous in warm, stagnant natural waters either free living or growing within amoebae.17 They proliferate in man-made water supplies with similar properties, eg water cooling towers, humidifiers, hot tubs, birthing pools, hot and cold plumbing systems and shower heads, etc.9 Legionellae favour growth temperatures in the range 20–45°C. Though clinical cases can occur in outbreaks associated with travel and as hospital acquired infection, most cases are sporadic and community acquired. Cases arise from exposure of the person to inadequately managed water supplies, including in the dental surgery. When investigating epidemiological risk factors associated with notified cases and outbreaks of Legionnaires' disease, Health Protection England include exposure to ‘dental treatment’ during the incubation period (2–14 days) as a pertinent risk factor on their case assessment protocols.13 Two large studies conducted before the current regulations were introduced showed the incidence of legionellae in DUWLs to be low (0.37% in London and Northern Ireland18 and 1.9% in the South-west of England19), but legionellae was also recovered from the cold water supply of three dental practices.18 Where legionellae are present in the water supply, raised legionella antibody titres can be found in the dental team, indicating occupational exposure.20,21 There is insufficient data to determine whether such exposures lead to infection such as Pontiac fever; but a death of a dentist was recorded in the USA following exposure to an unusual strain of legionella in his DUWL.22
Nontuberculous Mycobacteria (NTM) and pseudomonads are other pathogens that pose a transmission risk via DUWLs. NTM found naturally in potable waters are amplified 400-fold in DUWL and have been linked to two published cases of serious infection.23 Pseudomonas in dental primary care water lines in the UK can often be at high levels in untreated water lines.5 In the UK, Walker et al found that the most common species detected in their study was Pseudomonas species, isolated from 16% of untreated water lines.19
Martin24 demonstrated that Pseudomonas aeruginosa recovered from the dental abscesses in two immunocompromised patients treated in a dental school were identical to those found in the DUWL of the dental units where they were treated. In a follow-on prospective study, 78 non-compromised patients treated in one of six P. aeruginosa contaminated dental units were transiently colonized for 3–5 weeks with P. aeruginosa, but no infection ensued.24 But it is the death of an elderly lady in Italy following two visits to her dentist where she was exposed to legionellae in the DUWL that subsequently caused her pneumonia, the first proven case of dental-associated Legionnaires' disease, that is the most significant.7 The DUWLs were allegedly managed with a standard biocide (a hydrogen peroxide product), which has previously been shown to be effective. In summary, although the number of proven published cases of infection resulting from exposure to water from DUWLs is limited, these incidences are noteworthy cautionary tales that need attention to prevent further cases and improve our management of dental water supplies.
Criterion 2 of The Health and Social Care Act 2008: code of practice on the prevention and control of infections and related guidance (2015)25 sets out the criteria against which a registered provider's compliance with the management of DUWLs and water supplies will be assessed by the Care Quality Commission (England and Wales) for compliance with the law. Taking account published national guidance outlined in Approved Code of Practice. Legionnaires' Disease: The Control of Legionella Bacteria in Water Systems, usually referred to as L8,9 HSG27426 and HTM 01-05,3 the practice should have a policy for preventing contamination of dental unit water lines, including an appropriate water supply and maintenance schedules. Dental practices must, with the help of a Competent Person (Water Engineer3), prepare a legionella risk assessment and written control scheme (water plan) for Legionella prevention and control for their hot and cold water systems.9,25,26,27
Practitioners should be aware of the main risk factors for legionella contamination as control of these hazards determines the management of the water supply. The type of questions to consider when formulating a water plan are listed in Table 1.
|
|
If the answer to any of these questions in Table 1 is ‘yes’ for either the practice's plumbing or DUWL, as is the case in most dental practices, then these risks must be managed according to the regulations.9,3,27 Practices are under the regulations specified in the ACOP-L8 and are required to employ the services of a suitably qualified water engineer (Competent Person) such as a member of the Legionella Control Association or similar.9,27 The Competent Person‘s role is to provide professional specialized advice on preparing the risk assessment, writing the water management plan to control the risk, maintaining the cleanliness of the plumbing system and boiler and training the staff on taking sentinel water temperatures. An annual inspection by the engineer is recommended. The dental practice staff have the duty of recording and monitoring the water temperatures in the hot and cold water systems on a monthly, 6-monthly and annual basis, as set out in the water plan. Records must be kept for 5 years.3,9 A brief résumé of the basic principles on managing the risk from legionella in the practice's hot and cold plumbing are outlined in Tables 2 and 3. Readers are referred to HSG27426 and HTM 01-053 for a full description of the guidance and regulations. For readers in Scotland further information is also available from Scottish Health Technical Memorandum 04-0128 and the websites for Health Protection Scotland and Scottish Water. A discussion of the methods used to manage the DUWL is outlined in the next section.
| Regulations under ACOP – L8 |
Identify and assess sources of risk
|
|
Prepare a written scheme for preventing or controlling the risk
|
|
| Implement, manage and monitor precautions | |
|
Keep records of the precautions
|
|
|
Appoint a competent person with sufficient authority and knowledge of the installation to help take the measures needed to comply with the law
|
|
Hot water plumbing:*
|
|
Cold water plumbing:*
|
Dental unit water is both swallowed and inhaled by the patient. Therefore, the microbial quality of the water must take these factors into account. Health Technical Memorandum (HTM) 01-053 recommends that the bacterial count in water sampled from the DUWLs should have a bacterial total viable count (TVC) of ≤100–200 cfu/mL, which is comparable with drinking water standards in the European Union (≤100 cfu/mL) and USA (≤500 cfu/mL). Generally, the water entering the DUWL contains very few organisms: 0–100 colony-forming units (CFU)/ml. However, water exiting the handpieces may contain up to 10 000–1,000,000 cfu/mL. This is mainly as a result of the organisms that are shed from the bacterial biofilms growing within untreated lines; from the independent bottled water supply and from suck-back from the mouth due to negative pressure in the handpieces when drilling is stopped.
Although water can be taken from the mains supply (potable water), to achieve the specified water quality, dental units are usually fitted with a separate water reservoir that is independent of the public water supply. This allows dentists to have better control over the microbial quality of the water used in patient care by the addition of biocides and other methods to control contamination. In addition, they act as a type A air-gap, a physical gap that prevents back siphonage of contaminated water into the mains supply. Reservoir water bottles are recommended to be filled with freshly produced (less than 12 hours old9) reverse osmosis or distilled water. These purified waters are not sterile but are unlikely to contain legionella, NTMs and pseudomonads found in potable tap water.
The dental unit is classified as a medical device under the Medical Devices Directive 2002 and equipment used in practices must carry the European CE mark. It is well recognized that a combination of methods are required to prevent contamination and maintain water quality in the DUWL.3 This goal can currently be achieved using antiretraction and check valves, flushing, biocides and purified water in the reservoir bottle. Other methods are also commercially available.
This recommends dental practices to ‘Drain down, clean, flush and disinfect all system components, pipework and bottles TWICE daily, eg at the start and finish of each day’. Disinfection contact time is as recommended by the biocide (disinfectant) manufacturer. Biocides employed to prevent the growth of legionellae in DUWLs must comply with bacteriocidal activity specified in the standard BS EN 13623:2010 and before purchasing a DUWL biocide check in which the manufacturer states compliance with the standard on the accompanying data sheet.
Furthermore, HSG274 (part 3)26 recommends that ‘independent water storage bottles should be cleaned, rinsed with reverse osmosis or distilled eater, dried and stored dry and inverted overnight’.
These are not mandatory under the L8 ACOP9 or HTM 01-053 but are dictated by the individual dental practice's risk assessment, highlighting a possible hazard. Examples of possible hazards that would trigger microbiological measurements include, amongst others for example, technical factors causing an inability to comply with the regulations; after disruption in the service or physical changes noticed in the DUWL quality, such as foul taste or odour and cloudiness of the water.3. Dip-stick methods are not suitable for estimating legionella and water samples should be processed by a UKAS accredited water and environmental testing laboratory.3,18 The Laboratory will advise on sample collection methods but typically 1L of water is collected in a thiosulphate-coated bottle and transported directly to the laboratory for legionella testing, and enumeration of water-borne indicator organisms and aerobic colony counts obtained as specified in national standard operating procedures. Usually, the Laboratory will provide result interpretation guidance.
According to HTM 01-05, all DUWLs should be flushed for two minutes at the beginning of each day, prior to commencing treatment, and at the end of the day.3 Staff should be alert to malodour, cloudiness and bad taste imparted to the water by microbial contamination, which are particularly noticeable after periods of stagnation.3 They signal that conditions are appropriate to support the growth of legionella. The practice should seek advice on microbial sampling for legionella detection.3 Care should be taken by the operator to avoid splatter and aerosol exposure during DUWL flushing and masks and eyewear should be donned. Irregularly used dental units should be flushed on a routine basis (at least weekly) to prevent stagnation.3
This is a simple and efficient means of reducing the planktonic (suspended) bacterial load in the water line due to oral suck-back. Thereby helping to prevent cross contamination from a previous patient. DUWLs supplying handpieces, ultrasonic scalers and air/water syringes should all be flushed. Although flushing can reduce the numbers of bacteria in expelled water, the effect is transient and has no impact on the water line biofilm and may even increase the bacterial shedding momentarily as it disturbs the biofilm.29 Flushing also has the advantage of drawing up fresh biocide into the DUWL and thereby facilitating disinfection of the line.
All DUWLs should be fitted with check valves (non-retractable devices), to prevent suck-back (backflow/back-siphonage) of contaminants. If the dental unit takes water directly from the mains supply, then the unit should be fitted with a type A air gap. However, it is now known that the antiretraction valves can become a scaffold for biofilm formation and are very inefficient unless they are regularly maintained and replaced periodically.30 Antiretraction valves are also located in the dental handpieces. They prevent the re-aspiration of fluid contaminated with oral flora of the patient into the water line. Routine decontamination and periodic handpiece maintenance helps in maintaining the integrity of the handpiece anti-retraction valves.
Thermal disinfection and avoidance of temperature between 20–45°C, though highly effective as a control measure against legionella in the water distribution system, is not applicable to DUWLs. The ambient temperature in the DUWL is approximately 18–23°C.5
Therefore, in DUWLs, we are reliant on biocides and disinfectants to remove, inactivate and prevent formation of biofilm. The disinfectant must be active against the range of micro-organisms found in the DUWL, including legionella spp. Biocides can either be continuously infused into, or intermittently added to, the dental unit water by varying technologies. A huge variety of products are available for use as intermittent purges and continuous disinfection. A range of different disinfectants are deployed by manufacturers, eg alkaline or hydrogen peroxide, hydrogen peroxide/silver ions, peracetic acid formulations, Tetrasodium EDTA, chlorhexidine formulations, iodine, quarternary ammoniums and chlorine dioxide.31,32,33,34 Hypochlorite, popular for disinfecting DUWLs in the past, led to corrosion of handpieces and its use is mainly confined to ‘shock treatment’ for the eradication of microbiologically proven legionella contamination.7 For a detailed review of the adverse reactions reported for a range of different biocides see Coleman et al.31
Continuously infused biocides used in the water line must be non-toxic and safe if imbibed or inhaled by the patient and staff. Commercial products for continuous infusion are less concentrated than those sold for intermittent purging of the system.24
Water from DUWLs should never be used as an irrigant in procedures involving breaches of the mucosa and bone exposure – instead use a sterile water supply and delivery system or sterile saline.
Microbial point of use filters may be installed, for instance between the water line and the dental handpiece. These have no effect on the biofilm in the water lines, but will remove micro-organisms as the water is delivered to the patient. Disposable filters must be replaced daily, which can be expensive.3
Water purification systems treat the water coming into the dental unit (source water). They kill or remove micro-organisms by methods such as filtration, electrolyzed water or ultraviolet light. One advantage of these methods is that they may delay biofilm formation on water lines or synergize other treatment methods. As they are not discharged in the effluent water, they are non-polluting and less likely to select for antimicrobial resistant species than biocides used in DUWLs.35
Other, rather expensive methods, for delivery of quality water include the use of sterile water and autoclavable DUWL systems.
A ‘boil-water’ advisory notice is issued by authorities when the public water supply is likely to be contaminated with pathogenic organisms, eg Cryptosporidium, or the numbers of microbes in the public potable water supply system are above that which is compatible with health. A recent example occurred in Lancashire, UK during the summer of 2015. During such periods the following apply:
In the last two decades in response to the evidence base and publication of new regulations, dental practices in the UK and around the world have responded to the challenge of the DUWL goal set by the ADA in 1996 and are routinely treating their patients in a safe and hygienic environment.