Article


Patients with oral cancer may also be prone to co-morbidities, mainly:
Second primary tumours
Cancers may arise at multiple sites – either synchronously, or later (metachronously, ie at least six months after the primary tumour). Criteria for such SPTs (Figure 1) include:
SPTs may arise because of a genetic predisposition, or from the effects of lifestyle or possibly environment. There is some evidence that SPTs can share some, or even all, genetic markers with the index tumour, indicating that both tumours have arisen from a common clonal progenitor cell. Rare patients have a genetic predisposition to develop multiple tumours because they have an inherited mutation in one allele of the tumour suppressor gene p53 locus (Li Fraumeni syndrome). Patients suffering from Fanconi anaemia, a recessively inherited disease characterized by congenital anomalies and bone marrow failure, also have a predisposition to develop cancer, particularly squamous cell carcinomas in the head and neck and anogenital regions.
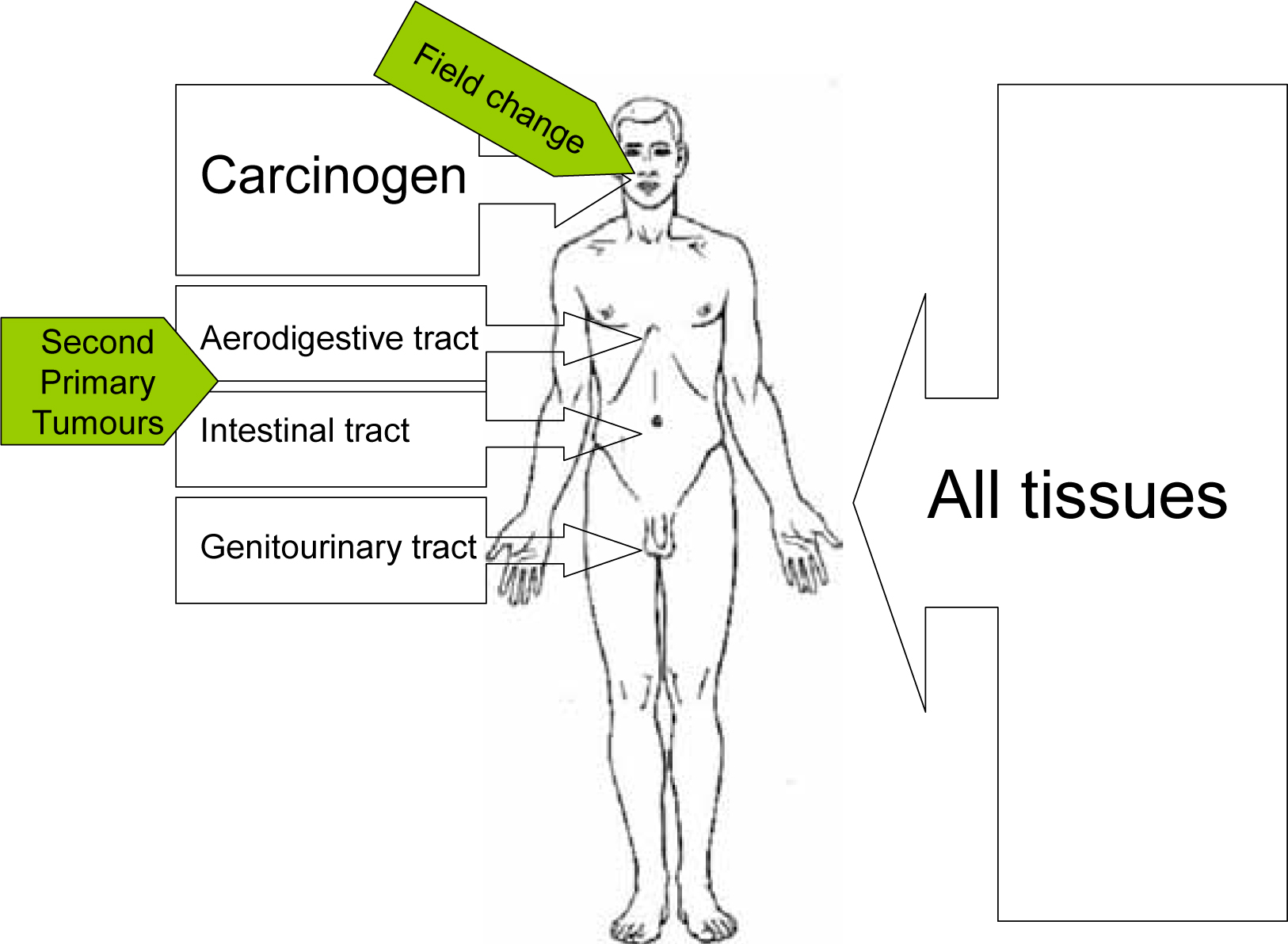
The lifestyle habits that are cancer risks expose not just the oral mucosa to carcinogens. A patient for example smoking tobacco, exposes the whole of the upper aero-digestive tract to carcinogens. This includes the mouth, nose, pharynx, larynx, bronchi and lungs, and the oesophagus. The carcinogens also circulate throughout the body to be excreted in urine and bile. Tobacco usage, not surprisingly therefore, can lead to head and neck, lung, oesophagus and bladder cancer. Patients with oral cancer may develop SPTs anywhere in the upper aerodigestive tract and, occasionally, one sees tumours elsewhere, as there is a slightly higher prevalence of bladder cancer, and of leukaemia.
Continued smoking increases both risk of SPT and death over that of former smokers compared with non-smokers. (Figure 2; Table 1).
| Within upper aerodigestive tract | Outside upper aerodigestive tract |
|---|---|
| Oral Cavity | Breast |
| Pharynx | Colon |
| Larynx | Ovary |
| Trachea | Pancreas |
| Lung | Bladder |
| Oesophagus | Stomach |
| Vulva | |
| Skeletal bone | |
| Lymphomas |
Finally, treatment of the primary cancer with radiotherapy (RT) in particular may lead to the development of a SPT, though most therapeutic RT is not associated with an increased rate. Indeed, the risk of SPTs may be reduced in patients treated with conventional RT. However, irradiation for retinoblastoma can lead to the development of jaw osteosarcoma. Concurrent chemotherapy (CTX) does not appear to change the risk of SPTs.
The prevalence of SPTs in oral cancer approaches 20% over nearly 10 years. Synchronous rates have been reported as 5% to 15%, while metachronous rates carry a continuous risk of 3% to 7% per annum, with 5-year rates varying between 15% and 25%. The reported percentages of SPTs depends on how they were detected: chest CT is more effective than panendoscopy, and 18-fluorodeoxyglucose (FDG)-PET-CT-scans may be particularly effective in detecting SPTs.
SPTs may develop more often when there is a tongue primary rather than a tumour elsewhere in the oral cavity or oropharynx, and are more common in younger patients. SPTs may arise at unfavourable sites, like lungs or oesophagus, but more often they arise in previously irradiated or operated areas. Therefore, the choice of treatment of the SPT may be influenced by the treatment of the index tumour and not all treatment options may be available for the SPT. Thus SPTs are not always to be treated conventionally – which may be why they may have a significantly negative impact on the prognosis in some studies. Furthermore, there may be a higher rate of late radiation-induced morbidity and impaired quality of life, eg speech and swallowing, after treatment of a SPT in a previously irradiated area.
The question arises as to whether SPTs are predictable and/or preventable.
Since SPTs may develop years after the index tumour, long-term (or even life-long) follow-up seems to be indicated, but the occurrence of SPTs cannot be predicted reliably in the individual patient and, despite recommendations, the real value of twice yearly chest radiography and bronchoscopy is questionable. New methods for detecting subclinical SPTs, such as serum biomarkers, photodiagnostic imaging, and molecular studies may change this negative view.
As to prevention, adverse habits should be stopped, but there are conflicting results about the effects on the frequency of SPTs. Chemoprevention is an attractive concept; high-dose isotretinoin given for 1 year decreased the risk of SPTs in a randomized trial, but tolerance was poor, with 70% patients requiring dose reduction. The development of SPTs has not been prevented by low dose isotretinoin, or by N-acetylcysteine, or etretinate, and alpha-tocopherol produced an unexpectedly higher occurrence of SPTs.
Other medical problems
Most patients with oral cancer are older adults, many exposed to tobacco, betel, alcohol or a combination, and often they are of lower socioeconomic groupings, sometimes malnourished. Co-morbidities are therefore commonly encountered. Cardiovascular co-morbidities include hypertension and ischaemic heart disease; respiratory problems include obstructive airways disease; and hepatic cirrhosis is common.
In one study, co-morbidity was present in 36% of patients. Mild decompensation was seen in 17%, moderate decompensation in 14%, and severe decompensation in 6%.
The most frequently observed co-morbidities – cardiovascular, respiratory, gastrointestinal and diabetes – show a significant relationship with short-term mortality. In several studies, severe co-morbidity was found to influence survival significantly and the evaluation of treatment effectiveness. In one study, 5-year survival in grade I and II ASA (American Society of Anaesthesiologists) patients was 44%, and, in ASA III and IV patients, it was only 16%. Also influenced by co-morbidities are the length of hospital stay, discharge destination and readmission rate.

