Abstract
This narrative review aims to highlight sources of the best available evidence and describe primary clinical trials currently taking place in primary dental care across the United Kingdom which will add to the evidence base.
From Volume 40, Issue 6, July 2013 | Pages 482-486
This narrative review aims to highlight sources of the best available evidence and describe primary clinical trials currently taking place in primary dental care across the United Kingdom which will add to the evidence base.

With the constant change in the dental landscape, it is becoming increasingly difficult for dental professionals to ensure that they are keeping up-to-date with legislation whilst also providing the best available care for their patients. At the same time, they could easily be pressured into believing that the latest dental material or product will solve all their clinical woes by the sheer strength of conviction of an advertising campaign or sales people. Patients' perceptions, priorities and expectations are also moving goalposts; with the widespread use of the internet, they can be quick to tell even the most experienced dental professional about the latest ‘breakthrough treatment’ that they expect to be provided. Many dental professionals will question where to seek reliable information, in a timely manner, without resulting in even more confusion.
The American Dental Association defines Evidence-based Dentistry (EBD) as:
‘an approach to oral healthcare that requires the judicious integration of systematic assessments of clinically relevant scientific evidence, relating to the patient's oral and medical condition and history, with the dentist's clinical expertise and the patient's treatment needs and preferences’.1
This definition was influenced by David Sackett's definition of evidence-based medicine, the key message of which is that Evidence-based Medicine (healthcare) ‘is the integration of the best research evidence with clinical expertise and patient values’2 (Figure 1).
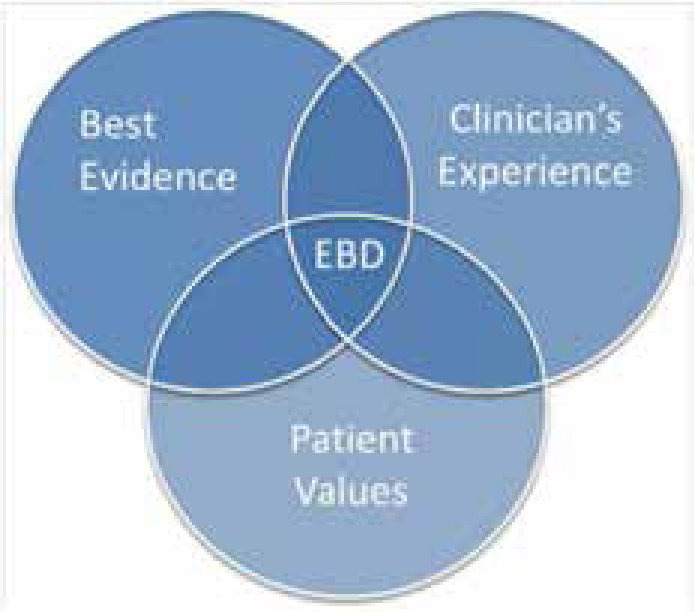
The concept of a clinician integrating his/her experience with the values of the patient and the best available evidence is not new. The methods of reviewing the best available evidence advanced in the 1990s with the systematic approach which was promoted by groups like the Cochrane Collaboration.
Whilst the principles of evidence-based dentistry have been widely accepted by the profession, the actual practicalities of implementing this philosophy can be challenging for the busy dental professional. This short narrative review aims to highlight sources of the best available evidence, and describe primary clinical trials currently taking place in primary dental care across the United Kingdom, which will add to the evidence base.
One of the pioneers of evidence-based medicine, Professor Archie Cochrane, was acutely aware of the challenges for clinicians as they attempt to keep up-to-date with advances in the evidence base. He commented in 1979:
‘It is surely a great criticism of our profession that we have not organized a critical summary, by specialty or subspecialty, adapted periodically, of all relevant randomized controlled trials’.3
When considering the vast amount of literature published on a weekly basis, it is impossible for an individual to filter all of the available studies to ascertain the best available evidence on a given subject.
Professor Cochrane promoted the systematic assessment of the available literature to guide clinicians, patients and policy makers in their decision-making processes. He stressed the importance of using evidence from randomized controlled trials (RCTs) because these were likely to provide much more reliable information than other sources of evidence.4
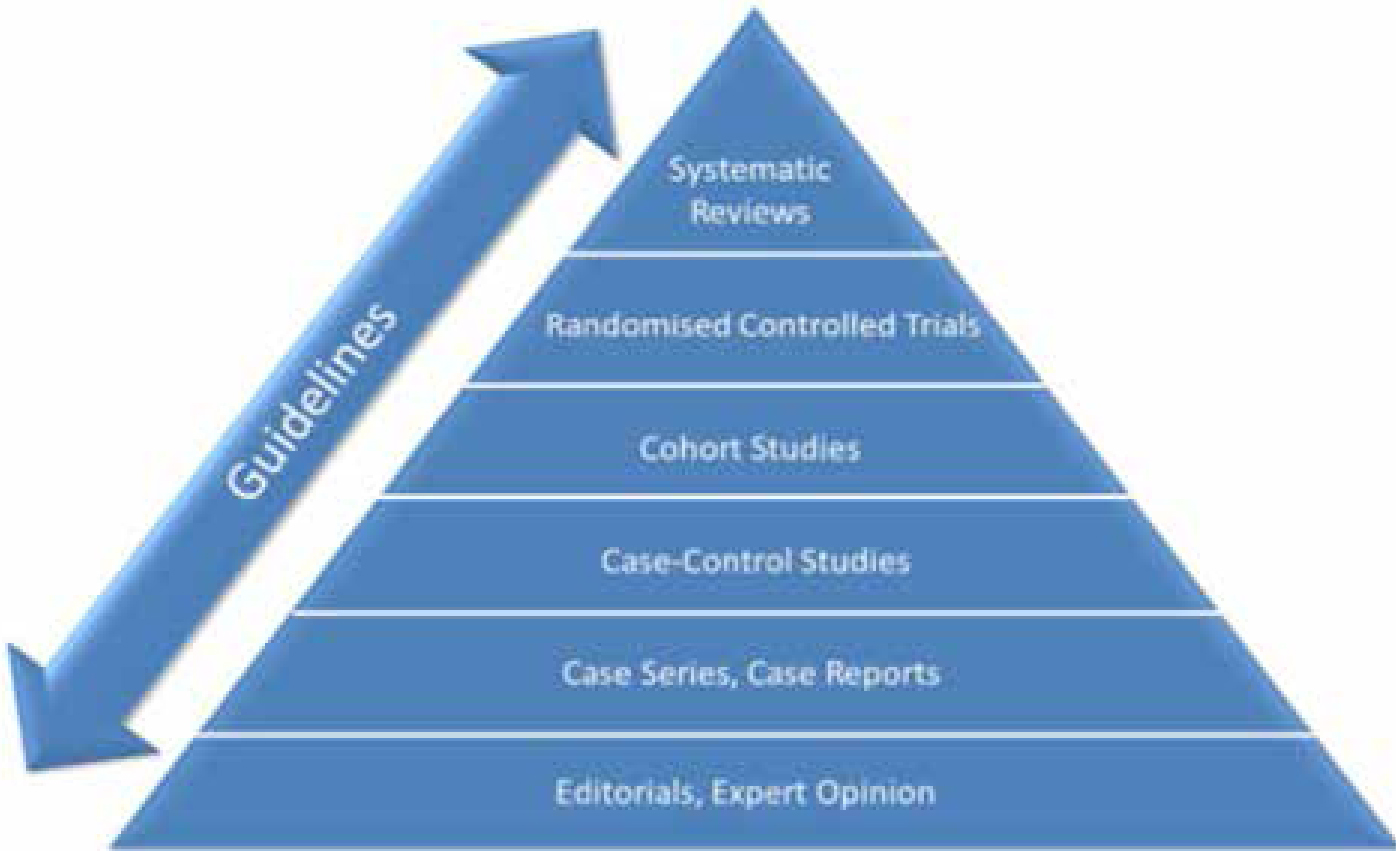
Systematic reviews can condense and appraise enormous amounts of literature, the results of which can then be used by clinicians, patients and policy makers to inform their decisions. The hierarchy of evidence is often quoted to demonstrate the relative robustness of the different research methods (Figure 2). We should be aware that the ‘Gold standard’ randomized controlled trial is not always the most appropriate methodology to answer a research question. Dental professionals will also be acutely aware that where RCTs are the most appropriate method, they have not always been conducted…yet. Figure 2 presents the hierarchy of evidence as it is most frequently presented but highlights the important roles of guidelines (guidance) which can include all of the different levels of evidence.
Clinical guidelines provide accessible recommendations on treatments based on the best available evidence. They are produced by multidisciplinary teams with increasing emphasis to ensure that they can be disseminated to, understood and utilized by patients, clinicians and policy makers. The National Institute for Health and Care Excellence (NICE) and the Scottish Intercollegiate Guidelines Network (SIGN) produce guidelines on all aspects of healthcare, amongst which are a few that address dental topics. (Guidelines freely available at http://guidance.nice.org.uk/ and www.sign.ac.uk/, respectively).
The Scottish Dental Clinical Effectiveness Programme (SDCEP) is an initiative of the National Dental Advisory Committee (NDAC) in partnership with NHS Education for Scotland (NES). SDCEP's primary aim is to provide user-friendly, evidence-based guidance to support dental teams' clinical and organizational decision-making and assist dental teams in providing the best possible dental care for their patients. SDCEP brings together the best available information that is relevant to oral healthcare priorities and presents guidance on best practice in a form that can be interpreted and implemented easily. The guidance recommendations may be based on research evidence, guidelines, legislation, policies and expert opinion, as appropriate to the subject. SDCEP guidance takes a variety of forms to suit the diverse topics being addressed.
Recognizing that publication of guidance alone may have a limited influence on practice, SDCEP also contributes to the development and evaluation of interventions to enhance the translation of guidance recommendations into practice via the TRiaDS (Translation Research in a Dental Setting) collaboration (www.triads.org.uk).5
SDCEP has provided dental clinical guidance on Decontamination (Cleaning and Sterilization of Dental Instruments); Conscious Sedation in Dentistry; Prevention and Management of Dental Caries in Children; Drug Prescribing for Dentistry; Emergency Dental Care; Oral Health Assessment and Review; Oral Health Management of Patients Prescribed Bisphosphonates (Figure 3). Most recently, SDCEP published Management of Acute Dental Problems. This guidance is of relevance to healthcare professionals in a variety of settings who might be required to advise or care for patients who present with a dental problem. SDCEP also maintains its online ‘Practice Support Manual’ which aims to support the organization, management and running of primary dental care practices. All SDCEP guidance can be sourced online from the SDCEP website (www.sdcep.org.uk/).

SDCEP is keen to employ novel approaches to guidance delivery and dissemination and therefore brought the popular ‘Drug Prescribing for Dentistry’ guidance to the smartphone/tablet platform by developing an App. It is organized into the same sections as the printed book but also includes a search function, bookmarking and direct links to the British National Formulary (BNF). Free updates to the App are provided to keep users up-to-date with each new edition of the British National Formulary for Children and the BNF. The latest publication, Management of Acute Dental Problems, has also been provided as a Web App – an interactive electronic version of the guidance that leads the user through the initial decision-making process with more detailed condition-specific advice for those who need it. These novel approaches for dentistry are part of the effort to make the best available evidence more accessible, in user-friendly formats, for dental professionals, patients and policy makers.
Another novel source for up-to-date, reliable evidence is the ‘The Dental Elf’ website and twitter account provided by Derek Richards and Douglas Badenoch. Derek is the Director of the Centre for Evidence-based Dentistry, Editor of the Evidence-based Dentistry journal and Consultant in Dental Public Health. He helped to establish both the Centre for Evidence-based Dentistry and the Evidence-based Dentistry journal. He has been teaching EBD for almost 20 years and now provides regular blogs which provide succinct, user-friendly summaries of the latest evidence-based literature. You can follow ‘The Dental Elf’ at www.thedentalelf.net/ or on twitter at https://twitter.com/TheDentalElf.
Although the guidelines, systematic reviews and summaries are making it easier for dentists to keep up-to-date, it is often the case that there is insufficient evidence on which to base recommendations or to inform decision-making. The Strategy for Oral Health research in Scotland highlighted that:
‘Many aspects of dental clinical practice still lack a robust evidence base and therefore an aspect of research that is being targeted by national and international funding bodies is clinical trials.’6
As a profession we still lack high quality evidence for some of the most basic treatments that we provide for our patients. The National Institute for Health Research Health Technology Assessment (NIHR HTA) programme is currently funding five dental clinical trials, based in the primary care setting, which should begin to address the paucity of high quality evidence available to patients, clinicians and policy makers.
NIHR HTA Filling Children's Teeth: Indicated or Not? (FiCTION) is a RCT investigating the effectiveness of three different approaches to managing caries in primary teeth:
NIHR HTA Northern Ireland Caries Prevention in Practice (NIC-PIP) trial is a RCT which will measure the effects and costs of a dental caries prevention regime for young children attending primary care dental services. (Protocol available – http://www.biomedcentral.com/1472-6831/11/27)
NIHR HTA Investigation of NICE Technologies for Enabling Risk – Variable – Adjusted – Length (INTERVAL) dental recalls trial is a RCT comparing the effectiveness and cost implications of three dental recall strategies: 24-month recall based on dentist assessed patient eligibility; risk-based recall based on NICE guidance; or traditional 6-month recall. (Protocol available – http://www.hta.ac.uk/project/2628.asp)
NIHR HTA Improving the Quality of Dentistry (IQuaD) is a RCT comparing the effectiveness and cost-effectiveness of oral hygiene advice and periodontal instrumentation (scale and polish) for dentate adults in dental primary care. (Protocol available – www.hta.ac.uk/2300)
NIHR HTA Seal Or Varnish? is a RCT to determine the relative cost and effectiveness of pit and fissure sealants and fluoride varnish in preventing dental decay. (Protocol available – www.biomedcentral.com/1472-6831/12/51).
The Universities of Belfast, Cardiff, Dundee and Manchester act as the trials' sponsors which involves overall responsibility for the initiation, management and/or financing of the clinical trials. The five trials are conducted in dental primary care whilst being designed and co-ordinated by large multidisciplinary teams of dental academics, statisticians, health economists, health psychologists, dentists, dental hygienists, dental therapists, dental nurses, administrators and members of the public, which bring together a wealth of experience and expertise. Staff members from 13 of the 16 dental schools in the United Kingdom play an active role in the organization and management of these trials.
When assessing the best available evidence, the literature must be both internally valid (good trial design, conduct and low risk of bias – guidance on the critical assessment of which has been covered in previous issues of Dental Update7,8,9) and externally valid (generalizable) meaning that the patients, intervention and context can be reproduced in usual care.10 Considering that approximately 90% of NHS healthcare is provided in the primary care setting, it is important that research is no longer predominantly reserved for secondary care or laboratory settings. Interventions should be tested on the patients and in the setting in which they are designed to be delivered. The long-term follow-up in all of these NIHR HTA trials will provide more meaningful results, giving a more realistic idea of the treatment effects that dental professionals and patients can expect in normal practice. The results are likely to impact beyond the United Kingdom.
Trials of this nature are never straightforward, but these NIHR HTA trials have been designed in a pragmatic manner with support and reimbursement provided to dental practices. The collaborations between general dental practices, health boards, research networks, universities and clinical trials units, with each contributing their particular experience and expertise, should enable the successful execution of these world leading dental clinical trials, thereby enhancing the evidence base around fundamental aspects of dental care.
The trials consider clinical, patient-centred and economic outcomes which will make them relevant to clinicians, patients and policy makers. Healthcare research has placed increasing emphasis on patient-centred outcomes, as opposed to the historic priority on clinical measurements, the relevance of which were not always obvious to patients or their dental professionals. At a time when patients should be taking more responsibility for their own health, this places their priorities and values at the centre of the investigations, which will hopefully improve oral healthcare in the areas with which they are concerned.
As a result of the sheer volume of literature published on a weekly basis, it is impossible for dental professionals to collect, appraise and integrate the evidence base by themselves. Guidelines, systematic reviews and high quality summaries present the current best evidence in user-friendly and manageable formats for dental professionals, patients and policy makers. These timely presentations should make it easier for dental professionals to integrate the best available evidence with their own clinical experience and their patients' values. It is only when we include all three of these factors that we are providing evidence-based dental care for our patients.