Abstract
Primate spaces are diastemata consistent with an inherent physiological pattern rather than functional adaptation. This article presents an atypically increased primate space only in the right mandibular arch.
From Volume 46, Issue 11, December 2019 | Pages 1072-1074
Primate spaces are diastemata consistent with an inherent physiological pattern rather than functional adaptation. This article presents an atypically increased primate space only in the right mandibular arch.

Primate spaces, those between the primary lateral incisor and canine in the maxilla and the primary mandibular canine and first molar in the mandible, are anthropometric diastemata that are consistent with an inherent physiological pattern rather than the result of functional adaptation.1 Approximately 60% of children have primate spaces in their primary dentition.2 Primate spaces are more commonly present in boys than girls and are often noted in the maxillary arch (83.7%) compared with the mandibular arch (61.2%).3
Although there is considerable variation in the presence or absence of primate spaces, they tend to remain stable following the eruption of the primary teeth, with little evidence of primary canine relationship changes in either arch.3 Primate spaces tend to occur more frequently and with wider mesio-distal dimensions in the maxilla compared with the mandible.4
Possible reasons for an enlarged primate space include individual variation,5 tooth size arch length discrepancy,6 or an obstructive pathology.
A 4-year-old Asian girl was referred to a paediatric dentistry clinic by a general dentist for management of an atypically increased primate space in the mandible on the right side. The patient was an only child and had a non-contributory medical history. There was no previous history of dental traumatic injuries or previous dental visits other than to the referring dentist.
Extra-oral examination revealed a symmetrical face and straight profile. Intra-orally the patient exhibited complete primary dentition with an increased primate space on the right-hand side, ie between the LRC and the LRD, creating asymmetry in the mandibular dental arch. Furthermore, a firm spherical raised area with pale overlying mucosa, which was not tender to palpation, was present (Figure 1). However, the other primate spaces, namely on the left-hand side and in the maxillary arch (mesial to the maxillary primary canines), were within normal limits.
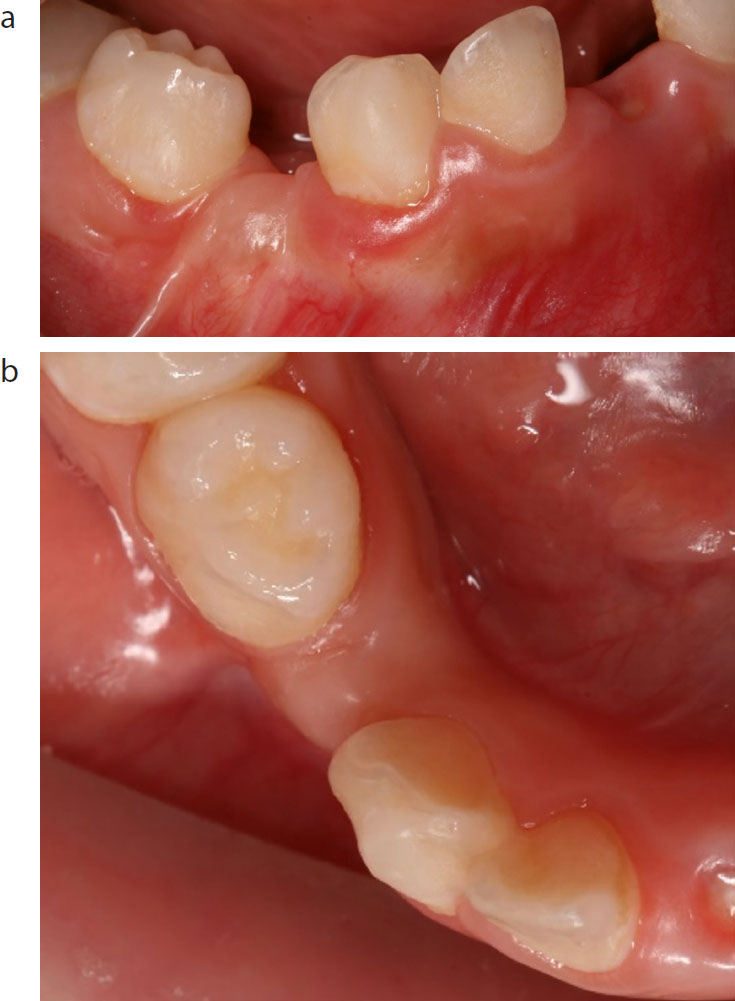
Based on the initial clinical presentation and non-significant medical or dental history, differential diagnoses, such as malignant neoplasm, developmental cyst, unicystic ameloblastoma, odontogenic keratocyst, infection or traumatic dental injury, were excluded. Although they are the second most common odontogenic cyst after radicular cysts, developmental cysts such as dentigerous cysts usually occur in the mandible and rarely involve primary teeth. They more commonly involve impacted unerupted permanent teeth, supernumerary teeth and odontomas. Odontogenic keratocysts commonly occur in the body or the ramus of the mandible, while unicystic ameloblastoma may cause expansion and destruction of the maxilla or the mandible.
Odontomas in the primary dentition are often asymptomatic and subsequently remain undetected. They could cause slow bony expansion as they develop. However, they are more commonly associated with non-eruption of the primary7 or permanent8,9,10 teeth rather than changing the width of the primate space.
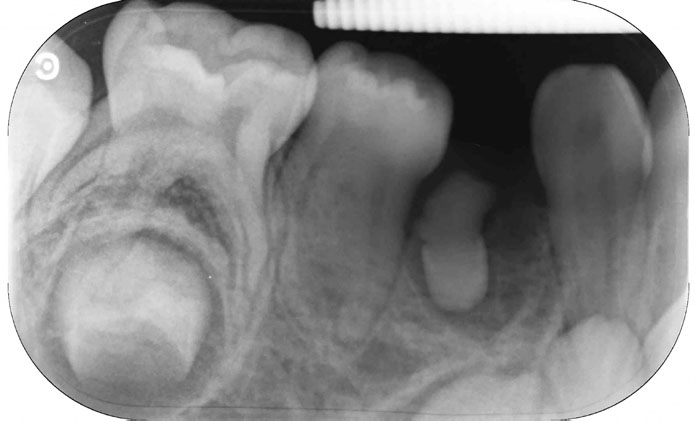
Radiographic examination (panoramic and periapical radiographs) revealed the presence of an inverted, conical-shaped, tooth-like structure in addition to the normal complement of 20 primary teeth positioned within the alveolar mucosa between the LRC and LRD (Figure 2). The tooth-like structure had a diffuse radiolucency associated with its entire periphery.
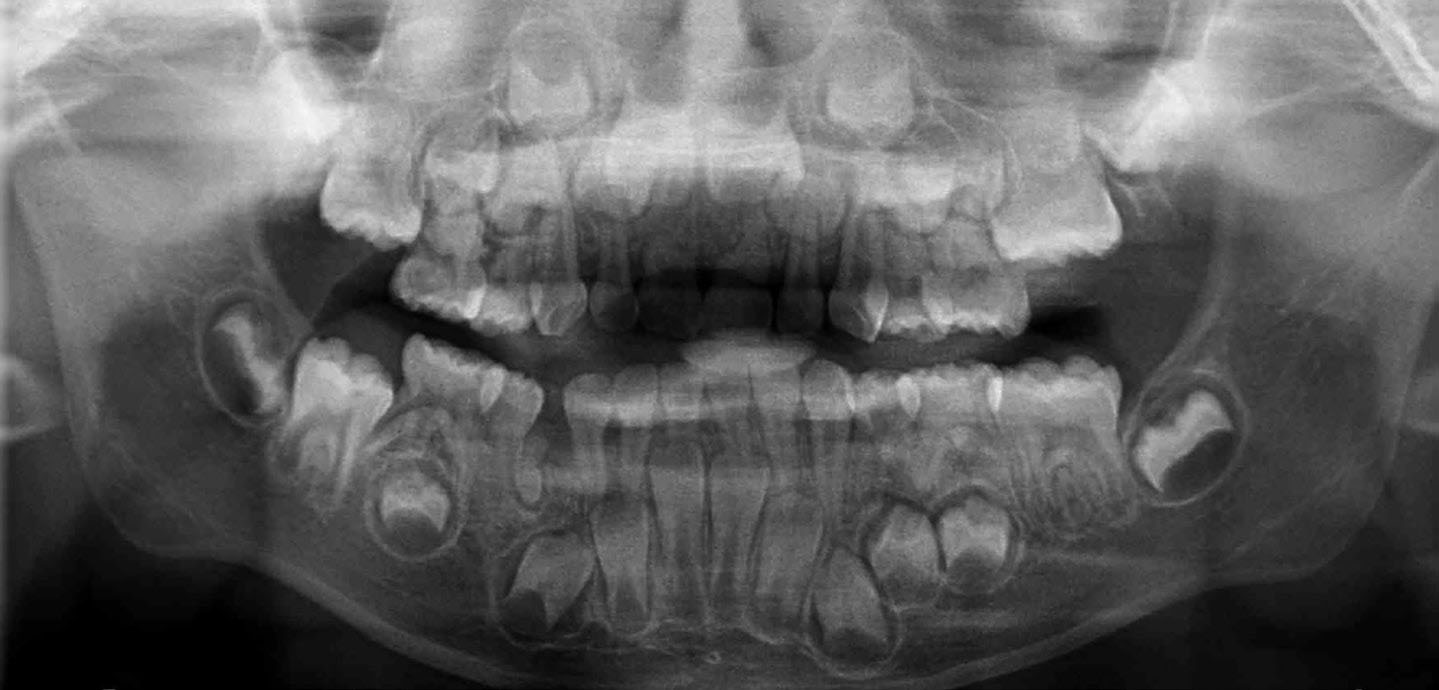
Panoramic dental radiography confirmed the clinical findings of a dental age corresponding with chronologic age, complete primary dentition and a supernumerary tooth in the primate space with an associated circumferential radiolucency (Figure 3). Interestingly, the LR4 appeared to be developmentally missing. Due to its size and inverted orientation, the supernumerary tooth was unlikely to erupt spontaneously and its radiographic appearance indicated possible cystic pathology associated with it.11 It was subsequently surgically removed under local anaesthesia without complication. The tooth was rudimentary in its crown and root morphology, with distinct enamel and dentine formation (Figure 4). The patient is being periodically reviewed.
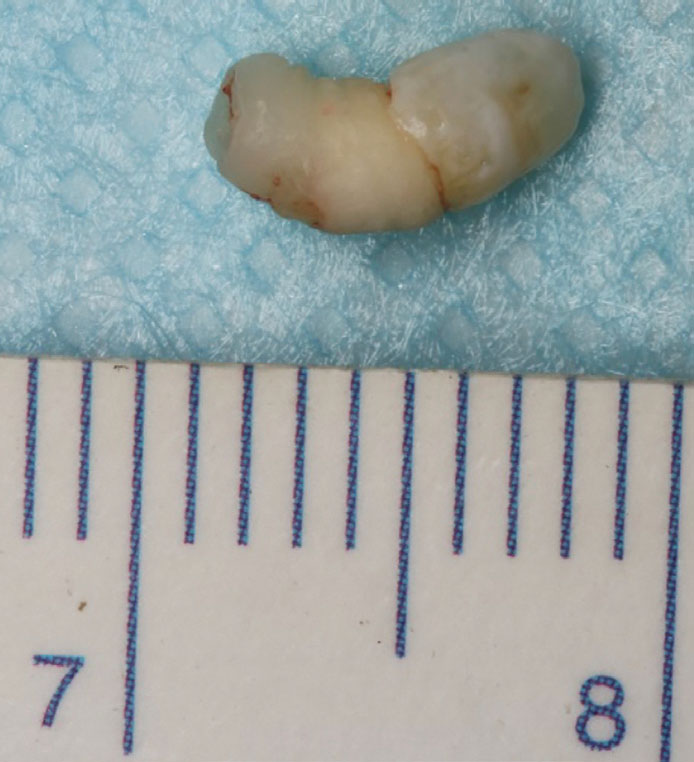
In this case, an inverted, tuberculate supernumerary tooth was observed radiographically within a mandibular primate space. Several theories have been proposed to explain supernumerary tooth formation. These include an atavistic principle, where mutations of certain genes that indirectly control the proliferation of the dental lamina cause increased expression of genes that are usually inactive, or of limited activity, but cause additional thickenings of the oral ectoderm and subsequent condensation of neural-crest-derived mesenchyme around the invaginating epithelium to form tooth buds.12
Signalling is crucial for determining tooth number, as studies have demonstrated in mice, where rudimentary or vestigial buds initially form in the toothless diastema region between the incisors and molars, a physiological space in mice, but degenerate without reaching the cap stage.13 Ahn et al demonstrated in a mouse model that inactivation of Wise (Sostdc1) leads to elevated Wnt/²-catenin signalling in the epithelium, allowing vestigial bud survival in a normally toothless diastema and subsequent development of supernumerary teeth within this space.14
In the present case, although very rare, hypo-hyperdontia is a possibility, ie if we consider the tooth-like structure to be a supernumerary tooth, then the patient also exhibits a developmentally missing tooth LR4, which is in the same region. Hypohyperdontia in the same region is extremely rare15,16 and not previously reported in the primate space region.
Another possibility is that the unerupted tooth-like structure is the mandibular first premolar. Ooe observed that, at 11 months, the germ of the mandibular first premolar, which begins developing postnatally on the lingual side of its predecessor in a position superficial to the occlusal surface of its predecessor, moves to a position between the roots of the premolar.17,18 The calcified premolar germ, located in the area enclosed by the roots of its predecessor, is rotated about 45 degrees, with the facial surface facing mesiobuccally. The lower second premolar is more rotated than the first mandibular premolar. This would explain the superficial position of the tooth-like structure within the alveolus of the primate space. Nevertheless, the stage of dental development of the tooth is more consistent with that of a primary tooth than that of a mandibular first premolar at age 4 years.15,19 Furthermore, the mandibular first premolar is less likely to be missing than the mandibular second premolar.16,20
The present case supports the hypothesis, proposed by Ahn et al in a mouse model, that it is possible to have tooth-like structures in the primate spaces and that tissue proliferation can potentially occur in any region of the jaws due to mechanisms of genetic control. It also highlights the importance of timely referral and imaging in assisting the diagnostic process and subsequent timely clinical management.