Abstract
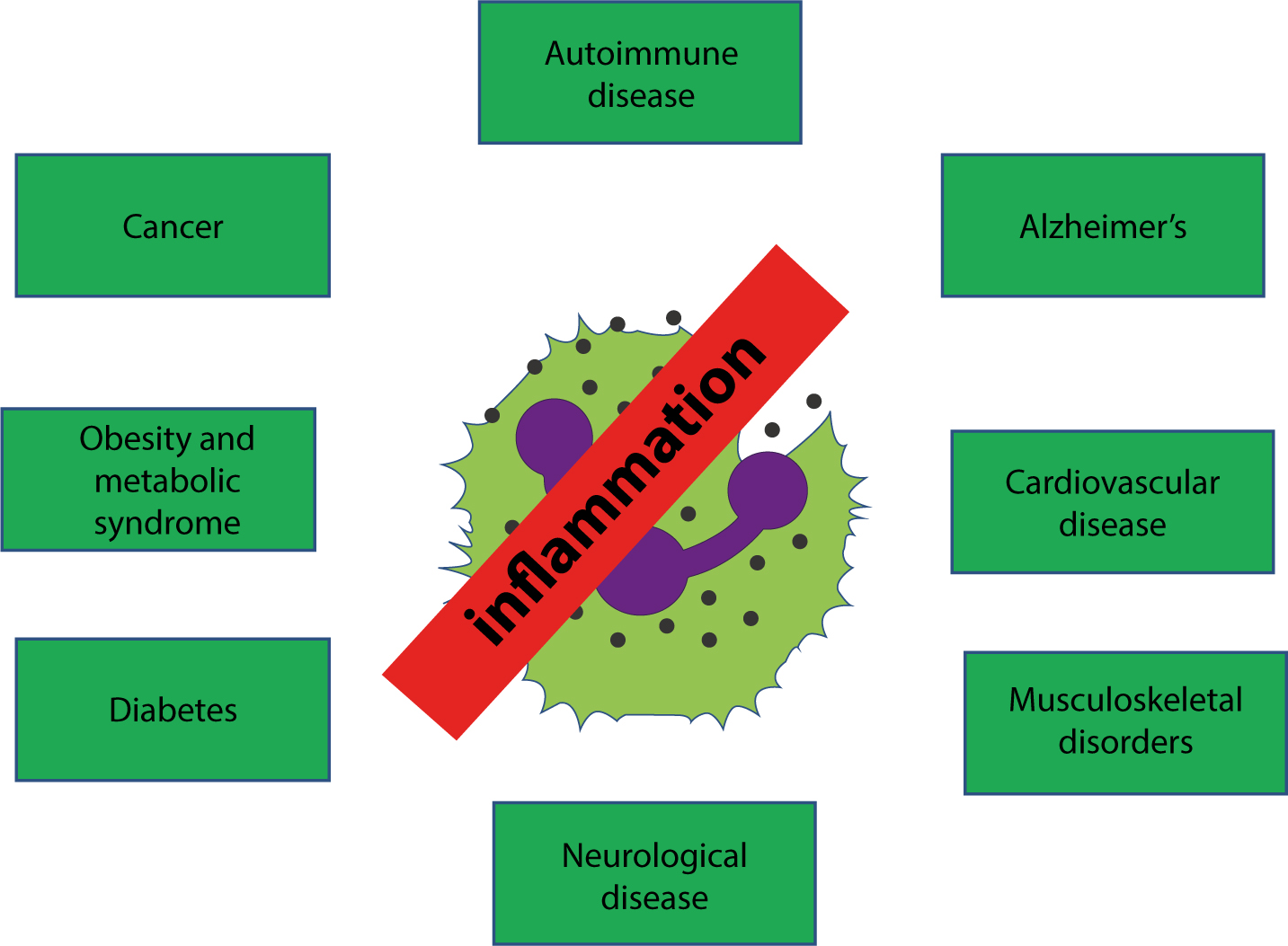
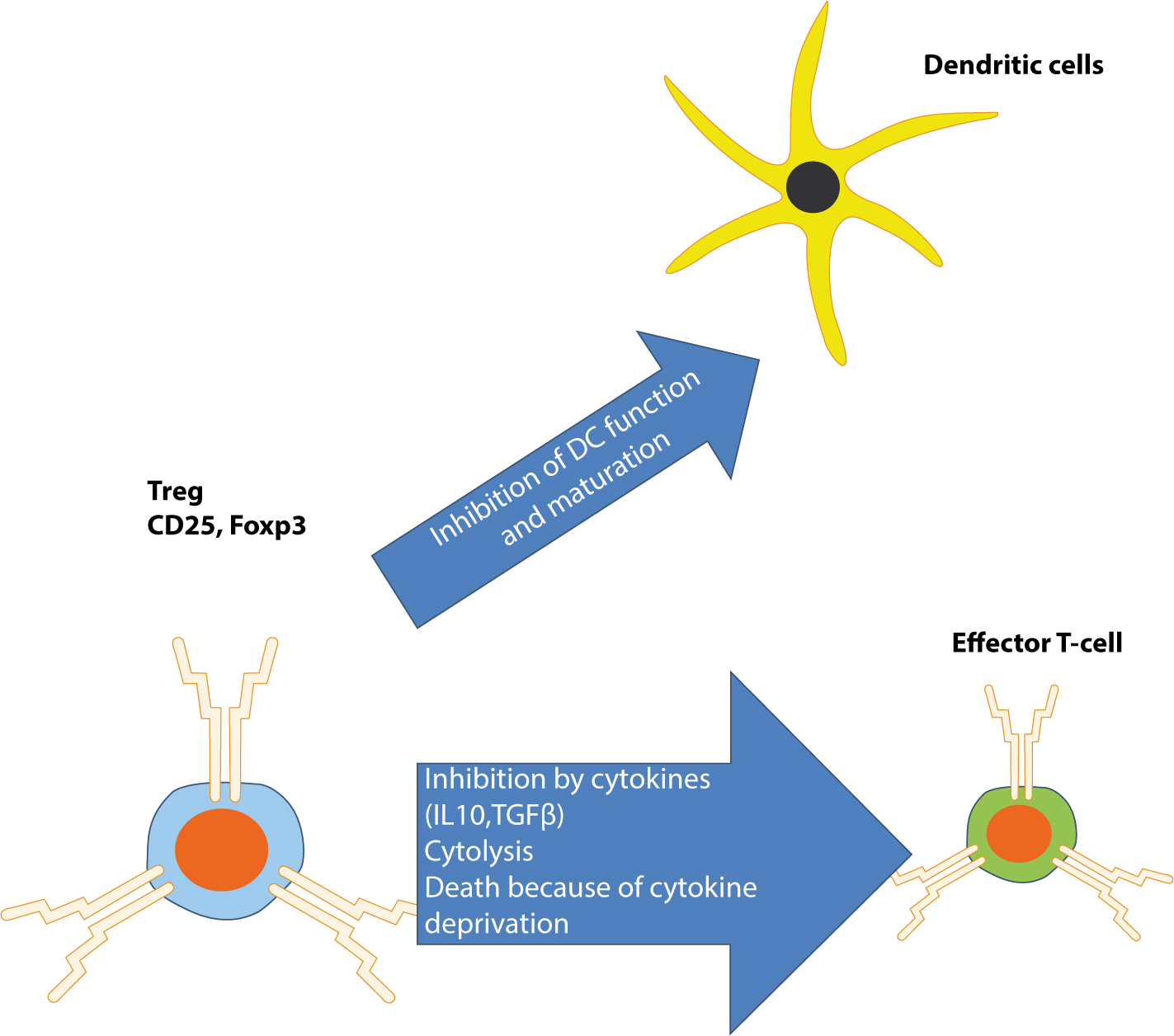
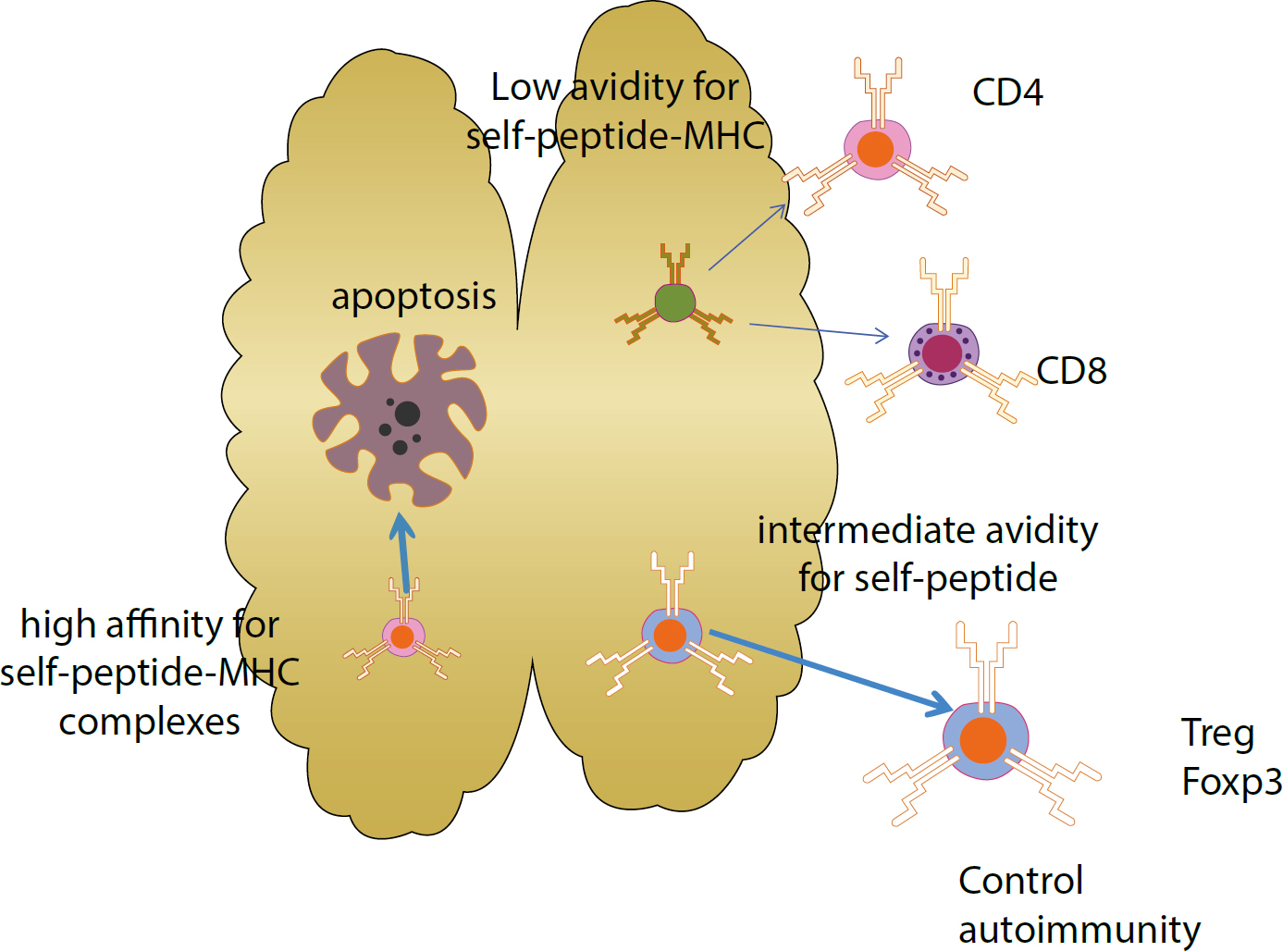
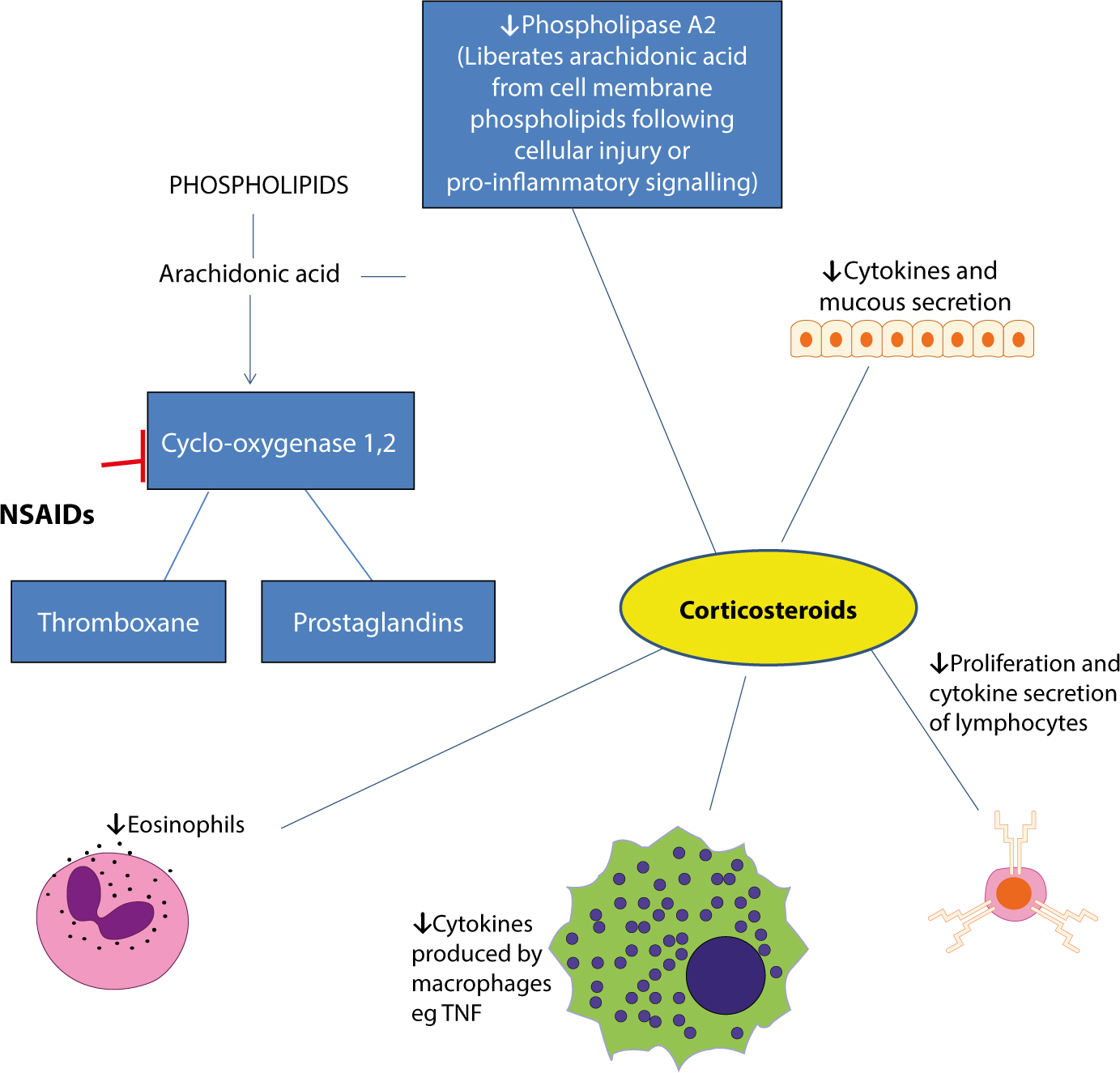
The immune system is the body's primary defence mechanism against infections, and disturbances in the system can cause disease if the system fails in defence functions (in immunocompromised people), or if the activity is detrimental to the host (as in auto-immune and auto-inflammatory states). A healthy immune system is also essential to normal health of dental and oral tissues. This series presents the basics for the understanding of the immune system, this article covering control of immunity and inflammation.