References
Taurodontism part 1: history, aetiology and molecular signalling, epidemiology and classification
From Volume 46, Issue 2, February 2019 | Pages 158-165
Article

Introduction and nomenclature
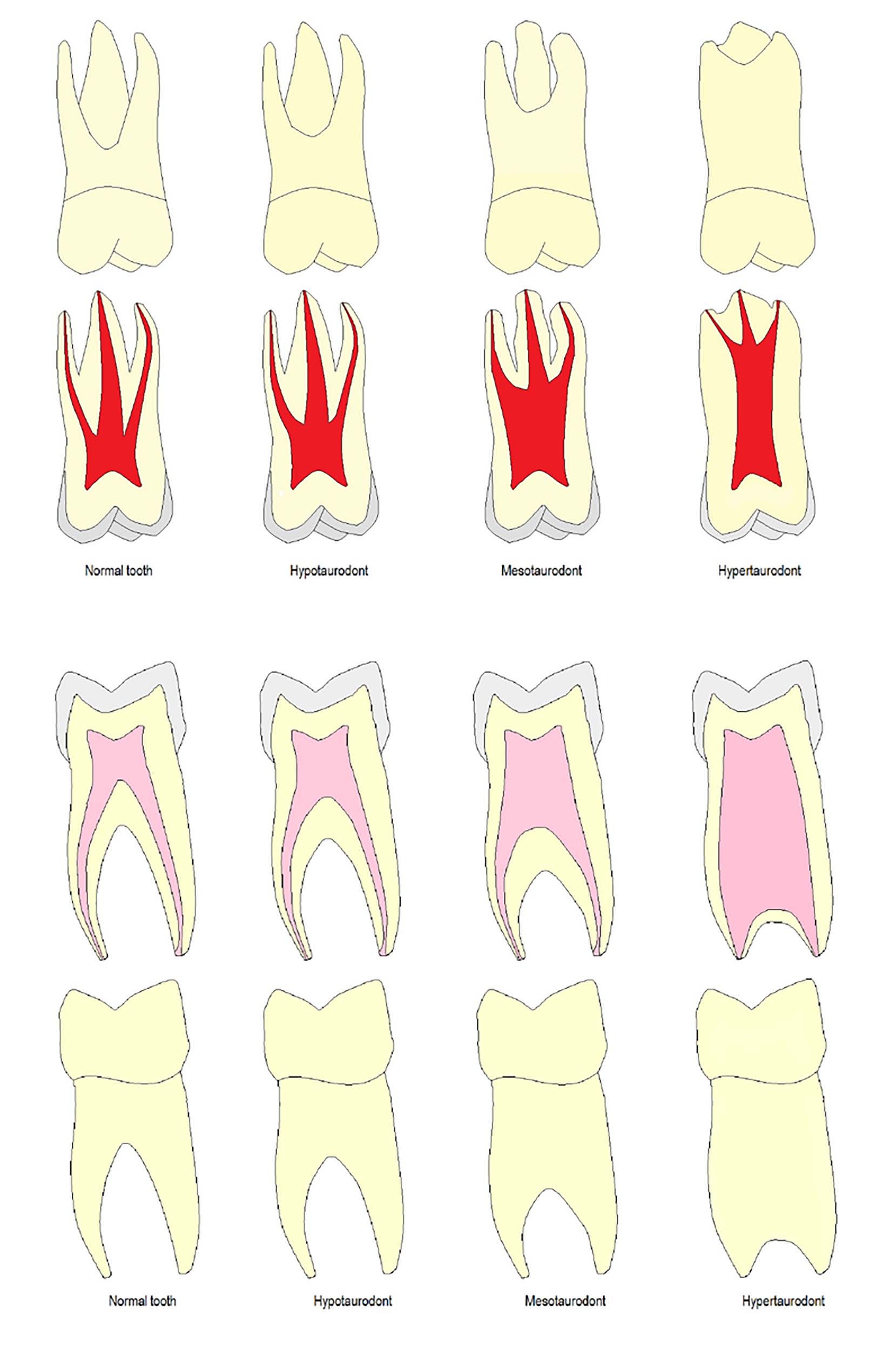
Initially, taurodonts were referred to as prismatic or cylindrical teeth before the term ‘taurodont’ was coined.1 In 1908, Gorjanovic-Kramberger was the first to describe Taurodontism.2 Later, in 1913, Sir Arthur Keith invented the term ‘Taurodontism’ for this morphological anomaly. The term taurodont is derived from a combination of Latin ‘tauros’ meaning bull and Greek ‘odonto’ meaning tooth, collectively known as a bull tooth.3 This condition is characterized by teeth lacking cervical constriction at the amelocementary junction with vertically enlarged pulp chambers, apical displacement of furcation and short roots (Figure 1).3
Historical background
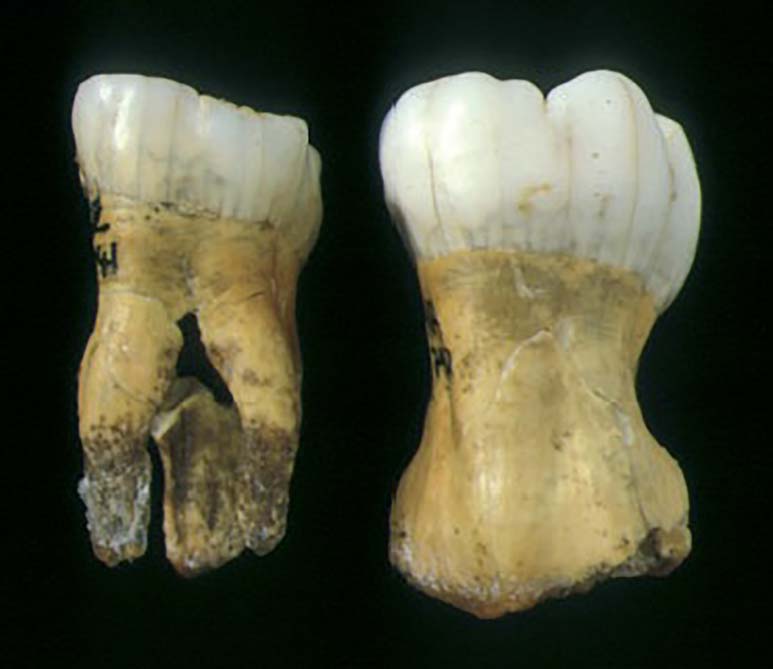
Taurodontism dates back to as early as the Neanderthals race (the Ice Age). This condition has been commonly observed in the fossil remains belonging to the Neanderthal hominids discovered in 1899 in Croatia (Figure 2). Neanderthals, a now extinct group of fossil hominins, were widespread across Europe from 200,000 until around 35,000 years ago.4 They were all known to have taurodont molar teeth − probably as a pleiotropic trait in common with many other dental and non-dental traits that were unique to them. There is a 70,000-year-old anthropological specimen belonging to the Krapina Neanderthals that shows taurodontism, making this anomaly a characteristic of a primitive pattern.5, 6
The study on a specimen found in Qesem Cave
In a recently published article, Fornai et al examined an anthropological specimen of deciduous second molar using contemporary methods.7 This well-preserved tooth was found in the Qesem Cave (QC) Middle Pleistocene hominin site in Israel, which is home to several dental remains. The site is associated with the Acheulo-Yabrudian Cultural Complex (dated to about 420,000 − 220,000 years ago). Researchers had studied this specimen previously, however, using conventional methods.8
Fornai et al used various methods to understand the inner and outer morphology of this deciduous second molar (the second study on this Qesem Cave specimen), labelled dm2-QC2.7 After gathering data from µCT images of the tooth, a detailed morphometric and endostructural comparative description of numerous dental aspects of a deciduous second molar specimen (dm2-QC2) was performed. These assessments included examination of dental tissues, descriptive investigation of inner morphology, and comparative 3D geometric morphometric investigation of numerous parts of the coronal structure. In addition to this, the researchers compared a deciduous second molar specimen with 44 specimens consisting of Homo erectus, Neanderthals and modern humans.
The results of their study showed that the deciduous second molar specimen showed thinner enamel, a large size with hypotaurodontism, a mid-trigonoid crest, mesio-distal elongation of the enamel dentine junction and mild crown expansion on the distal side. These aspects brought the deciduous second molar specimen close to the Neanderthals, while present day humans also share some of its features.
Mechanism of root formation
Root formation originates from designated cells of epithelial and mesenchymal origin. The inner enamel epithelium gives rise to the Hertwig's epithelial root sheath (HERS), which forms the initial outline for the root's morphology. In multi-rooted teeth, this root sheath further invaginates at the furcation region, resulting in the formation of multiple roots. Delay or failure of this step causes disturbances in root formation and leads to several morphological aberrations, such as additional roots and root canals, pyramidal roots and taurodontism.9
After the process of invagination, the Hertwig's epithelial root sheath interacts with the underlying mesenchymal cells (predecessor of pulpal cells). The epithelial cells then signal pulpal cells through molecular signalling. This leads to the differentiation of pulpal cells into odontoblasts, which lay root dentine.10
The aetiology of taurodontism
The conventional belief behind the aetiology of taurodontism remains in delayed or failed invagination of the diaphragm of Hertwig's epithelial root sheath, which results in the formation of short roots, enlarged pulp cavities and lack of cervical constriction.3, 11
Molecular signalling and taurodontism
As per widely held belief, the cause of taurodontism is known to be due to delayed or failed invagination of the Hertwig's epithelial root sheath (HERS). However, the signalling mechanisms that trigger this delay or failure are unknown.12
In this regard, research has demonstrated a possible role of a signalling molecule, Wnt10a, in root formation, when expressed in cells of dental papilla (mature odontoblasts and mesenchymal cells). Hence, defects in this signalling molecule might lead to a delayed or failed invagination of HERS. This delayed or failed invagination of HERS causes formation of a long root trunk, apically displaced furcation and short roots, resulting in taurodontism.13 In a recent study on molecular genetics of null mice, Yang et al probed into the functions of this signalling molecule, Wnt10a, which could be responsible for tooth agenesis, malformed crowns and taurodontism. A null mouse or a knockout mouse is a genetically engineered mouse. It is generated when a current gene is inactivated (knocked-out) by either disruption or substitution with a synthetic part of DNA. Yang and his team studied the effects of loss of Wnt10a function in null mice and in six human families with non-syndromic tooth agenesis.14 The next few paragraphs will discuss this study.
Gene-targeted embryonic stem cells were used to generate Wnt10a null mice by the Mouse Biology Program for the Knockout Mouse Project (KOMP) and involved several steps of genetic engineering. When the null mice reached the 16th week, four mouse heads were delivered for evaluation. These included three archived Wnt10a null mouse heads and one archived wild-type head. At first, samples were carefully sliced to create hemi-mandibles. The specimens were studied using radiography, microscopic photography and micro-computed tomography. Whereas in human subjects, 5 ml peripheral blood or 2 ml saliva was received and genomic DNA extraction was carried out. The extracted DNA was then checked using spectrophotometry and later examined using Whole-exome analyses and mutational analyses of Wnt10a. It is beyond the scope of this article to describe the results of this study (for more detail, refer to the article by Yang J et al.)14 In summary, the authors observed the following dental phenotype in Wnt10a null mice:
In six human families with non-syndromic tooth agenesis, the following Wnt10a defects were noted:
Root resorption and other pathological features found in null mice with Wnt10a defects were absent in dental phenotypes noted in human candidates. The authors concluded that the signalling molecule, Wnt10a, plays an important role in furcation and root formation, especially of multi-rooted teeth.14
Other potential causes are, delay in the growth of the transverse processes15 or odontoblastic deficiency during root dentine formation.16 Possible external factors include disruptive developmental homeostasis,17 administration of high dose chemotherapy,18 bone marrow transplantation19 and osteomyelitis20 (Table 1).
| Internal Factors | External Factors |
|---|---|
| Failure of invagination of the diaphragm of Hertwig's epithelial sheath | Osteomyelitis |
| Odontoblastic deficiency during root dentine formation | Chemotherapy (high dose) |
| Genetic (higher number of sex chromosomes) | Bone marrow transplantation |
| Interference in the epitheliomesenchymatose induction | Disruptive developmental homeostasis |
| Changes in the mitotic activity of cells of the developing teeth | Delay in the growth of the transverse processes |
Prevalence in population and ethnic distribution
Although a rare condition, the prevalence of this morphologic anomaly ranges from 0.1% to 60%.6, 21, 22, 23, 24, 25, 26, 27, 28 This wide range is probably due to disparities between various population groups and different diagnostic standards set in studies. Based on individual population groups, some prevalence data are given in Table 2. Taurodontism is widely distributed among different ethnic groups, however, it is more frequently seen in natives of Central America, Australia and Alaska.29
| No. | Population Type Based on Region | Reported Prevalence (%) |
|---|---|---|
| 1. | American population21 | 0.9 |
| 2. | German population22 | 2.5 |
| 3. | Jordanian population23 | 8 |
| 4. | Western European population24, 25 | 10 |
| 5. | South African population26 | 30 |
| 6. | Chinese population27 | 46.4 |
| 7. | Senegalese population28 | 48 |
| 8. | Australian population5 | 60 |
| 9. | Prevalence range globally (approx) | From 0.1 to 60 |
Prevalence in teeth and gender predilection
Taurodontism is seen primarily in permanent teeth and, to some extent, in deciduous teeth. Mandibular molars show the highest incidence of taurodontism,30, 31, 32 followed by maxillary molars and premolars. This seems debatable since a study has shown permanent maxillary second molars to have the highest prevalence for taurodontism.33, 34
Taurodontism can occur in multiple teeth or can be isolated to a tooth with unilateral or bilateral distribution. Several studies show that this condition occurs equally among men and women.35, 36, 37 However, in a study carried out on a German population, women had more taurodonts than men, although this finding was not statistically significant.22 Similarly, only one Chinese study has shown taurodontism to be more prevalent in women.27
Classification
Taurodonts are sub-classified based on their degree of pulpal floor displacement towards the apex, as hypodont, mesodont or hyperdont, whereas a cynodont is a normal tooth (Table 3, Figure 1). The Taurodont Index (TI) was invented by Keene36 and later modified by Shifman and Chanannel.38 This index was developed to assess the severity of taurodontism through a quantitative radiographic assessment. In this index, the height of the pulpal cavity (V1) is divided by the distance from the lowest point of the roof of the pulp cavity to the longest apex (V2) and then multiplied by 100. If the value is more than 20%, taurodontism is present (Table 3).
| Degree of Taurodontism | Types of Taurodontism | Value (V1/V2x100) |
|---|---|---|
| Mild | Hypotaurodontism | 20%–29.9% |
| Moderate | Mesotaurodontism | 30%–39.9% |
| Severe | Hypertaurodontism | 40%–75% |
| Absent | Normal tooth (Cynodont) | <20% |
Taurodontism Index values (Figure 3)
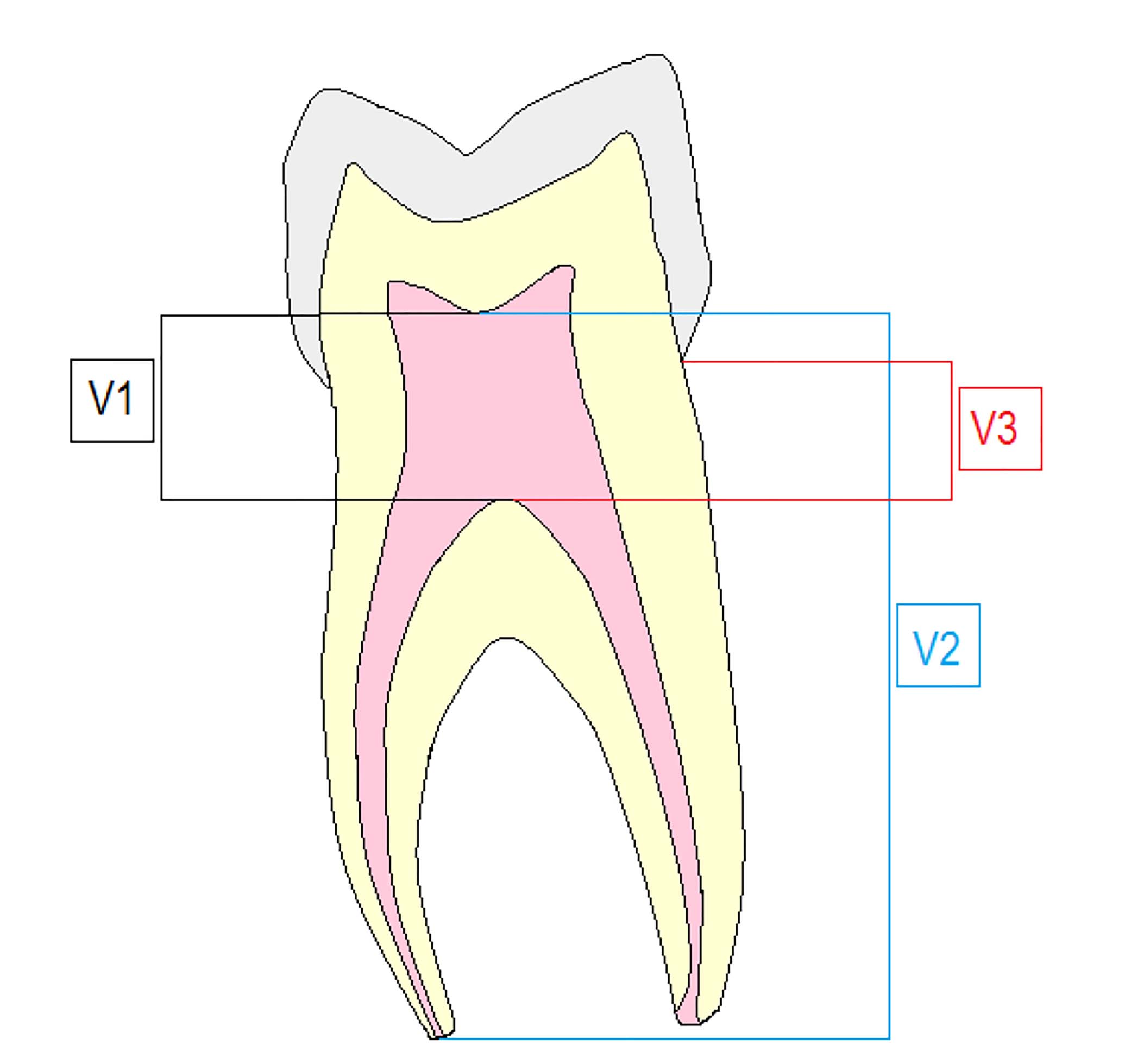
Taurodontism Index values are given in Table 4. A formula for calculating severity of taurodontism (Figure 3) is: V1/V2 x 100 = TI severity (%)
| Value | Description |
|---|---|
| V1 | The lowest roof point to highest floor point (the height of the pulpal cavity) |
| V2 | The lowest point of the root of pulp cavity to the longest apex |
| V3 | Cemento-enamel junction to chamber floor |
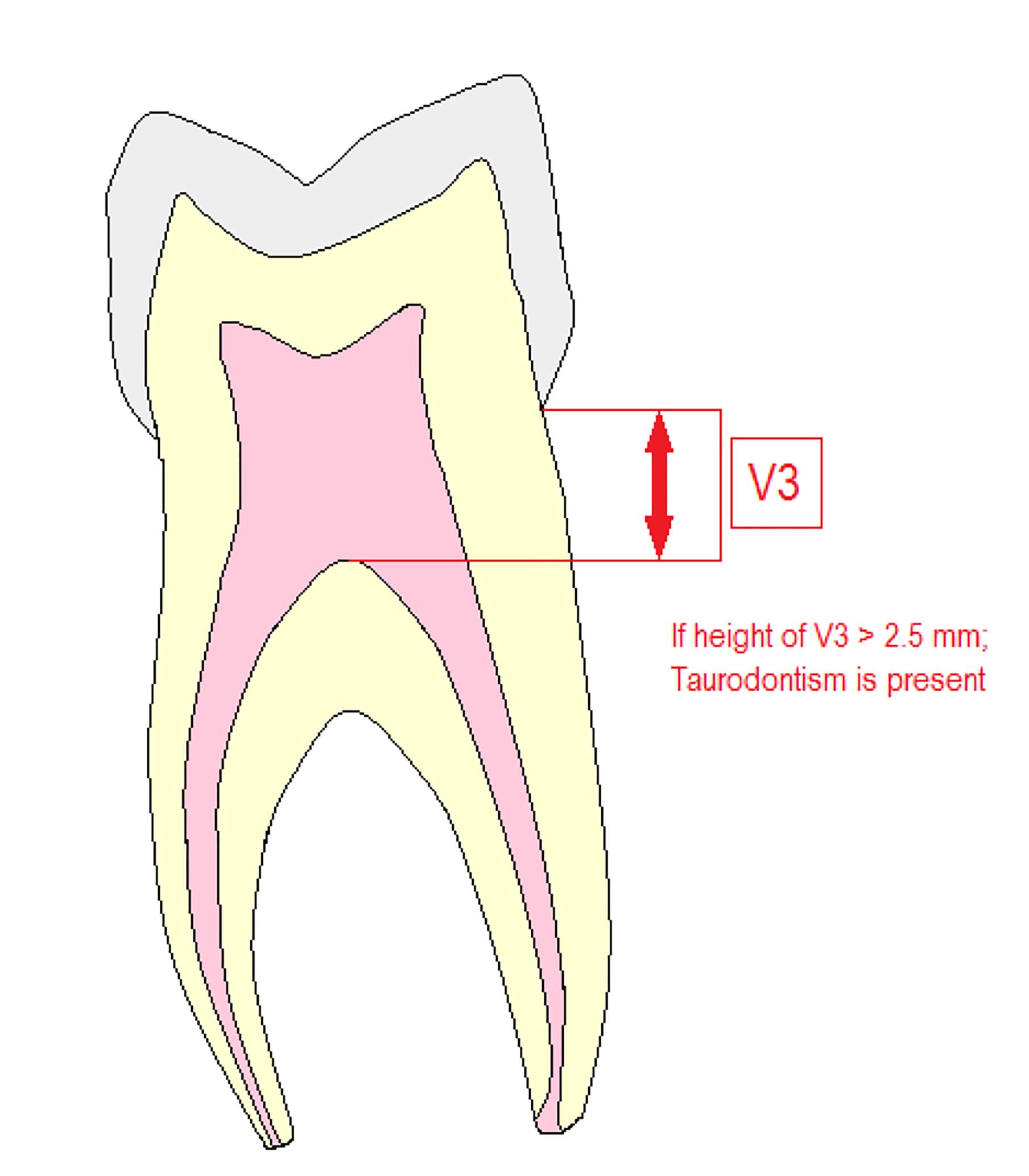
Another way of determining taurodontism is to measure the arbitrary distance from the highest end of the pulp cavity to the cemento-enamel junction (CEJ), (V3). Shifman and Chanannel suggested that the arbitrary distance should be more than 2.5 mm for taurodontism to be present (Figure 4) and for a tooth to be a taurodont, the ratio from the height of the pulp cavity to the distance between pulpal roof and longest root apex should be at least 0.2.38
Modification to the original Taurodontism Index (TI)
The original taurodontism biometric index was invented by Keene.36 This index considered internal morphology, unlike the index proposed by Shaw that relied upon external morphological landmarks and measured crown-body to root ratio (CB:R) (Table 5).26, 39 Yet, it had errors that affected the classification of taurodontism because of alterations in the morphological landmarks. This was due to physiological age-related changes in the tooth structure. Shifman and Chanannel38 later modified this index into a more accurate and objective assessment method, which is still used today.
| Classification | Crown-Body: Root Ratio (CB:R) |
|---|---|
| Cynodont (Normal tooth) | <1.10 |
| Hypodont | 1.10–1.29 |
| Mesodont | 1.30–2.0 |
| Hyperdont | >2.0 |
The modified index denotes the severity of taurodontism by calculating values and categorizing them into preset values. An index value of 20% to 29.9% is hypotaurodontism, a mild form. A value of 30% to 39.9% is mesotaurodontism, a moderate form. Whereas a value of 40% to 75% is diagnosed as severe taurodontism known as hypertaurodontism. A value less than 20% is a normal tooth (Table 3). Even so, this index does not take into account following factors:22
Values for assessing severity of taurodontism
Values for assessing severity of taurodontism are given in Table 3.
Summary
In conclusion, taurodontism can occur in both maxillary and mandibular teeth, especially permanent molars, and is a chance finding during radiographic examination. Based on the literature review, it does not to have a gender predilection. There could be a possible role of the Wnt10a molecule in the development of taurodontism, as discussed in early sections of this review article.
The second article in this two part series will discuss clinical elements, such as biomechanics of taurodontic teeth, differential diagnosis, disorders similar to taurodontism and its associated medical syndromes and will also mention preferred imaging methods, clinical implications and their management.

