References
Gingival retraction techniques: a review
From Volume 45, Issue 4, April 2018 | Pages 284-297
Article

The aesthetics and longevity of restorations is significantly dependent on gingival and periodontal factors. The intimate interaction between the restorations and the surrounding soft tissues means that all procedures performed should keep the health of the gingiva and periodontium under consideration. Restorations placed in close proximity to the soft tissues sometimes require consideration of subgingival margins,1 otherwise the subsequent restorations may have a high chance of failure.2,3 Also, in directly placed adhesive restorations, isolation for subgingival placement requires control of crevicular fluid. Without this important step in the restorative procedure, optimum qualities of the adhesive restorative material cannot be assured.4 In order to record subgingivally placed margins, the adjacent soft tissue needs to be retracted and displaced adequately for the impression material to penetrate and capture, not only the features of preparation and finish line, but also some unprepared tooth structure apically.5 The sulcular width should be at least 0.2 mm so that the impression material does not tear or distort when removed from the sulcus.6 Moisture control during composite placement also requires isolation in such a way that the properties of composite are not compromised.7
The application of gingival retraction in various dental procedures is summarized in Table 1. This review will focus on the assessment of the tissues to be retracted as well as the procedures and products that can be employed to ensure adequate gingival retraction.
| 1. Isolation of cavity prepared close to the gingival margin |
| 2. Control of haemorrhage during restorative material placement |
| 3. Diagnosis of subgingival caries |
| 4. Recording subgingival margins during impression for indirect restorations |
| 5. Protection of the gingiva during preparation of tooth for direct or indirect restoration with subgingival margins, including implant-supported restorations |
| 6. Better visualization of the preparation margins |
| 7. During crown lengthening procedures |
| 8. Helps visualize margins and remove excess cement during final seating and cementation of indirect restorations |
| 10. Removing excessive gingival tissue |
Pre-retraction assessment of gingival tissues
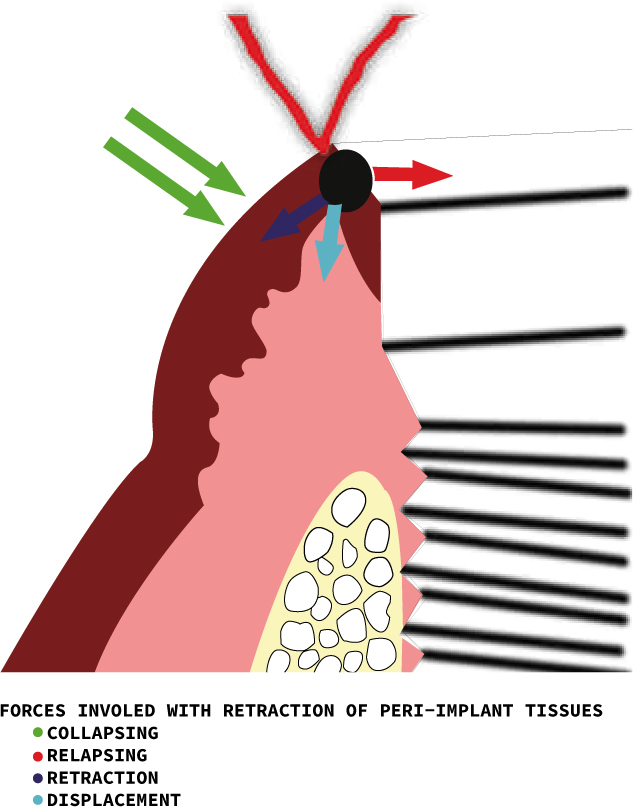
Before any gingival retraction is contemplated, and ideally before any restoration with subgingival margins is planned, it is important to assess the gingival tissues and adjacent supporting structures thoroughly. This is essential because the placement of subgingival margins and the procedures undertaken to record these margins can damage the delicate gingiva. If the tissues are already compromised, any traumatic retraction method can further damage the tissues.8,9 When a gingival retraction technique is utilized, forces act in four directions on the gingival tissues. These are the retraction, displacement, collapsing and relapsing forces (Figure 1).10
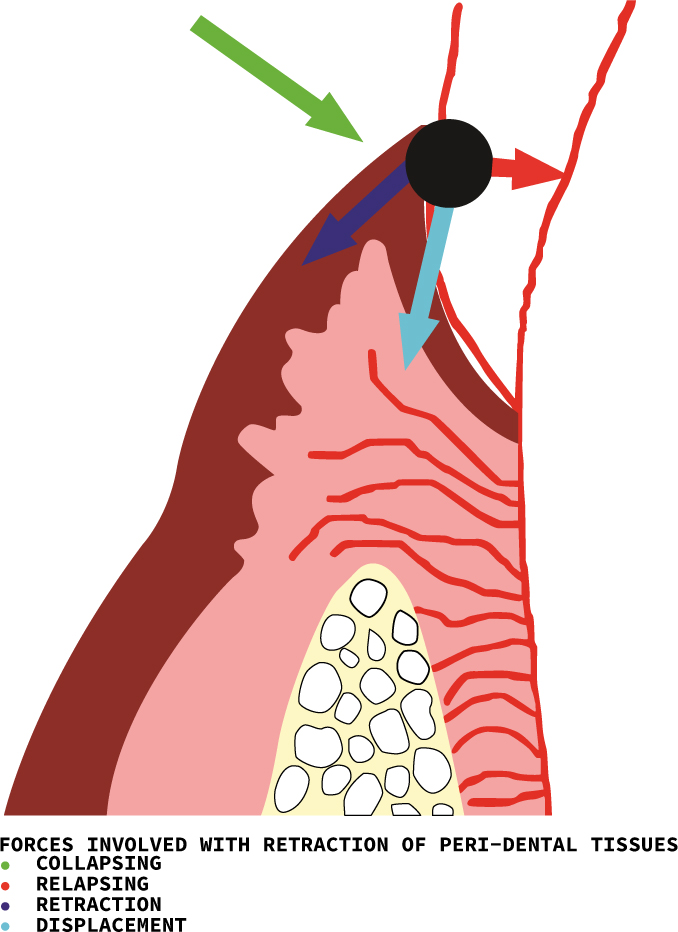
When the soft tissues are healthy, with a fibre-rich connective tissue supporting the delicate epithelium, there is less chance of damage to, and collapse of, the gingiva when the retracting agent is removed. The following evaluation should be undertaken prior to retracting or displacing the gingival tissue.
Clinical assessment
The gingival tissues intended to be retracted should be pink in colour and firm. The gingival biotype should be identified, which is a useful indicator of the behaviour of the gingiva to operative procedures and gingival displacement. Gingival tissue has been described as mainly having thick or thin biotype, although any variation of the two can be seen clinically, and their characteristics are given11 (Table 2). Thin gingival biotypes are more likely to be adversely affected with a subgingivally placed restoration and hence, the treatment and restoration should be planned accordingly.11 The contour, consistency and any pain originating from the gingiva or supporting tissues should be evaluated. There should be minimum or no bleeding on probing. Bleeding indicates inflamed and damaged gingiva, which is difficult to isolate and is more likely to get damaged during the retraction and displacement process. Gingival indices can be utilized to identify healthy and diseased gingival tissues.12
| Thin Gingival Biotype | Thick Gingival Biotype |
|---|---|
| Tissue thickness <1.5 mm | Tissue thickness >2 mm |
| Highly scalloped gingival architecture | Less scalloped, flat gingival architecture |
| Thin, narrow inter-dental papilla | Thick, wide inter-dental papilla |
| Associated with narrow triangular teeth | Associated with wider square teeth |
| Underlying bone thin | Underlying bone thick |
| More prone to recession | More prone to pocket formation |
| Less resistant to trauma | More robust and resistant to trauma |
Gingival sulcus is also an important parameter to assess the placement of restoration margins. Margins placed too deep in the sulcus require more retraction of the gingival tissue, resulting in damage to the supporting structures of the tooth. If margins are to be placed subgingivally, it is recommended to place the margins 0.5−1mm below the gingival margin,13 especially where the probing depth is less than 1.5 mm, and ideally to control the apical extent of the preparation so as not to encroach on the epithelial and connective tissue attachment. Although studies have indicated that there is no accelerated bone loss with subgingival margins, there can be recession of the soft tissues with the unaesthetic exposure of the gingival margins.14
Radiographic assessment
Both peri-apical and bitewing radiographs can be utilized to assess inter-proximal bone levels and crestal bone height, as well as infra-bony pockets and boss loss. Unsupported soft tissue, with underlying deficient bone, has a greater chance of recession when gingival tissue is traumatically displaced to record subgingival margins.5
Methods of gingival retraction
Mechanical methods
These techniques involve physically retracting and displacing the soft tissues, making space for the impression material to reach the recess of the subgingival preparation, as well as providing haemostasis and controlling crevicular fluid during direct composite restoration placement or cementation of deep subgingival indirect restorations. These include the following.
Matrix band and wedges
Matrix bands can provide retraction of gingiva and isolation when used for cervical or subgingival restorations. Wedges placed inter-proximally physically depress the gingival for retraction, and can protect the gingiva during preparation of the tooth.15
Gingival protector
This is a small instrument with a crescent-shaped tip, which can be placed and adjusted according to the contour of the gingival tissues physically to protect the gingiva during preparation of tooth structure close to the gingival margin (Figure 2). They are useful during subgingival cavity removal and cavity preparation, finishing veneer and other indirect restoration margins and to check the proper seating of crowns with subgingivally placed margins.15

Rubber dam
The use of heavy, extra heavy and special heavy rubber dam, together with specialized clamps (eg Ferrier 212, Schultz, Brinker's clamp B5, B6), help to retract and protect the gingival tissues during the preparation of the tooth as well as providing isolation for subsequent restoration placement. Inversion of the dam also aids in isolating the gingival tissues. With the help of modified trays, impressions can be made with the clamps in place but it is difficult and cannot be applied to full mouth impressions. Also, some components of the rubber dam, like sulfide, can retard the setting of polyvinyl siloxane elastomeric impression material and, therefore, the two should not be used together.
Copper ring technique
This method involves the use of a copper band or ring, with the gingival margins festooned according to the gingival contours. This is useful for impression of an indirect restoration with subgingival margins, where the copper band is filled with modelling compound or elastomeric impression material, and seated on the prepared tooth along the path of insertion. This method physically displaces the tissue, which stays retracted when the copper band is removed, so that the subsequent impression records the subgingival tooth structure. This technique may result in damage to the gingival tissues during placement, as the assembly is difficult to remove once set due to the presence of undercuts.16
Anatomic retraction caps
The retraction caps follow the same principle as the copper bands, except that they are pre-shaped, for easy placement between adjacent teeth and, once in place, the patient bites on it. The physical pressure arrests haemorrhage and opens the sulcus for the final impression.
Retraction cords
They are considered the most popular method for displacement of gingival tissue. According to fabrication, they can be knitted, twisted or braided and can also be classified as impregnated (if already containing medicament or haemostatic agent) or non-impregnated. Any configuration of the cord can be used according to the clinician's preference, as all different types of cords lack standardization in size. They are, however, colour-coded and vary in diameter (usually indicated by numbers 000, 00, 0−3), to be used in different clinical situations and gingival sulcus depths. They come pre-cut (according to the diameter of teeth) or can be dispensed from a container or a clicker.⁵ Ideal properties of retraction cords include:17
Braided cords have a tight weave, and hence are easier to place into the gingival sulcus without fear of fraying. They also have good absorbency if used with medicaments. Braided cords have a greater tendency to push out of the sulcus from one point when pressure is applied along another segment.18
Knitted cords are popular and have interlocking loops which helps to shape and bend the cord passively during placement in the gingival sulcus (Figure 3). This configuration also prevents the cord's displacement once the adjacent segment is being pushed into the sulcus. This type of cord has a tendency to compress while being placed and, therefore, a slightly thicker size should be selected to compensate for this.18 Also, a non-serrated and smoother instrument should be used for their packing as they have a tendency to unravel if used with serrated instruments.5 Twisted cords have the greatest tendency to untwist and fray during placement in the sulcus. They are not routinely used in favour of braided and knitted cords.19

Special cords
One product, the Stay-put retraction cord, has a thin wire incorporated into the centre of the retraction cord (Figure 4). Available as both plain and pre-impregnated, this cord offers the advantage of maintaining its shape once inserted inside the gingival sulcus. The pliability of the cord also makes it easier to place in the sulcus and the cord can also be pre-shaped. The pre-impregnated cord uses aluminum chloride, which diminishes the chances of cardiovascular symptoms. It comes in four sizes, according to width (0−3), and can also be used in conjunction with compression caps, which come in regular and anatomic shapes. The anatomic compression caps have a semi-circular shape on the facial and lingual surfaces, hence they can be placed on adjacent teeth for retraction. After the cord is placed, the compression cap is placed on the tooth and the patient is asked to bite. This helps in further retraction of the sulcus.18

Chemo-mechanical methods
This method employs the retraction cord with use of a chemical or a medicament. A wide variety of materials have been used in conjunction with gingival cords. The cords may be pre-impregnated with these chemicals or plain retraction cords may be soaked in them before placement. The main function of all these chemical agents is to arrest haemorrhage and decrease the leaking of crevicular fluid, while the cord physically displaces the gingival tissues. They can be vasoconstrictors that cause contraction of the blood vessels, Astringents™ that contract the gingival tissue or chemicals that cease bleeding by haemostatis and coagulation. Some products are available in gel or liquid formulation, which can be directly syringed into the gingival sulcus for arrest of bleeding and crevicular fluid. This can be followed by placement of the cord. The chemicals used for this purpose can be classified according to their mode of action (Table 3).
| 1) VASOCONSTRICTORS |
| a) Epinephrine |
| b) Nor–epinephrine |
| 2) BIOLOGIC FLUID COAGULANTS |
| a) 15.5–20% Ferric sulfate |
| b) 100% Alum |
| c) 15−25% AlCl3 |
| d) 10% Aluminium potassium sulfate |
| e) 15−25% Tannic acid |
| 3) SURFACE LAYER TISSUE COAGULANTS |
| a) 8% ZnCl2 |
| b) Silver nitrate |
Epinephrine has been the most popularly used chemical with which retraction cords were impregnated,20 although its use for this purpose has decreased overtime.21 It is most commonly used as 8% racemic epinephrine, but other concentrations have also been used.22 Retraction cords are either dipped in epinephrine or come pre-impregnated. Because of the high vascularity of the gingival tissue, the systemic effects exerted by epinephrine have been a cause for concern, especially if the gingival tissues have been lacerated.22 The systemic effect of epinephrine has been described as ‘epinephrine reaction’ or ‘epinephrine syndrome’ and is associated with the use of epinephrine-soaked retraction cords. This is characterized by tachycardia, increased blood pressure, nervousness, anxiety, increased respiration and post-operative depression. One study indicated that there was almost 50 times more epinephrine in 1 inch of retraction cord as in 1 cartridge of 1:100,000 epinephrine.23 This is a clear indication of how cautiously epinephrine impregnated cords must be used in patients with significant cardiovascular history. Some of the effect exerted by epinephrine can be avoided by using in diluted form and for the minimum amount of time needed for retraction. Some studies have even demonstrated that there is no significant difference in degree of retraction while using plain and epinephrine impregnated cord.24
Astringents™ have gained in popularity as adjuvants in gingival tissue retraction due to minimal systematic side-effects. They not only produce haemostasis, but also cause tissue retraction by decreasing the elasticity of the collagen fibres in the gingival tissues surrounding the tooth.19 This helps in keeping the sulcus open even after the removal of the retraction cord. They also decrease the oozing of crevicular fluid from the gingival sulcus, which improves visibility, makes a good impression more likely and also improves bonding for adhesive restorative procedures. Ferric sulfate (15.5−20%) is commonly employed as a coagulant while performing associated gingival displacement.5 The problems associated with using ferric sulfate is the removal of smear layer if placed for more than 10 minutes.25 This can cause sensitivity in patients after the procedure. Also, ferric sulfate can form a residue on the tooth surface, which interferes with the impression setting and can also discolour the dentine, due to its high iron content.26 Furthermore, if a composite restoration is planned, the residue can interfere with the bonding of composite to the tooth.27 If ferric sulfate is to be used with the retraction cords, the sulcus should be washed out after removal of the cord and prior to impression-taking.28
Another agent used for haemostasis is 20−25% aluminum chloride. It has been found to be least irritating to the gingival tissues but also results in the removal of the smear layer and dentine etching.5
Alum and aluminum sulfate are considered to be the safest astringents because they do not have any significant systematic effect, but they are also less effective at controlling haemorrhage and crevicular exudates.17 They have limited use in gingival retraction methods.
Zinc chloride (bitartrate) and silver nitrate both physically causing haemostasis and precipitation of protein on the mucosal surface, resulting in coagulation. Zinc chloride is available in 8% and 40% concentrations but its use has been associated with soft-tissue injury and hence is no longer recommended.29 Studies have described ophthalmic or nasal decongestants as potential vasoconstrictive and haemostatic agents used in conjunction with retraction cords due to their active components, like tetrahyrozoline or oxymetazoline, which are sympathomimetic amines. These are mild compounds with local vasoconstriction and minimal systemic effects.30 One study described them to be safer than 25% aluminum chloride for epithelial cells. These medicaments are still not approved for clinical use as gingival haemostatic agents.
Cord packing instrument
Some instruments have been marketed as retraction cord packers, developed specifically for the insertion of the retraction cord into the gingival sulcus. But many clinicians use a variety of instruments for this purpose. It is important that, whatever instrument be used, its working end should be thin enough to pack the cord into the sulcus efficiently, but not sharp enough to initiate bleeding from the sulcus wall or cause any perforation (Figure 5). The instrument can also be dual-ended, with working edges at different orientations to facilitate the insertion of the cord encircling the tooth, without having to change hand positions or instruments. This design also prevents hindrance in the field of vision. The working ends can be smooth or serrated, depending on the preference of the operator (Figure 6). The smooth round-ended instrument is mostly used for packing twisted cord while the serrated type is used for the braided variety.5 The serrated ends work by preventing the slippage of the cord during placement, but have the disadvantage of causing fraying of the cord if not used cautiously. For inter-proximal cord packing, a periodontal probe can be used as gingival tissues are thin and delicate in this area. For thin gingival biotype, a flat plastic instrument can work well for placing the retraction cord without damaging the delicate tissue.
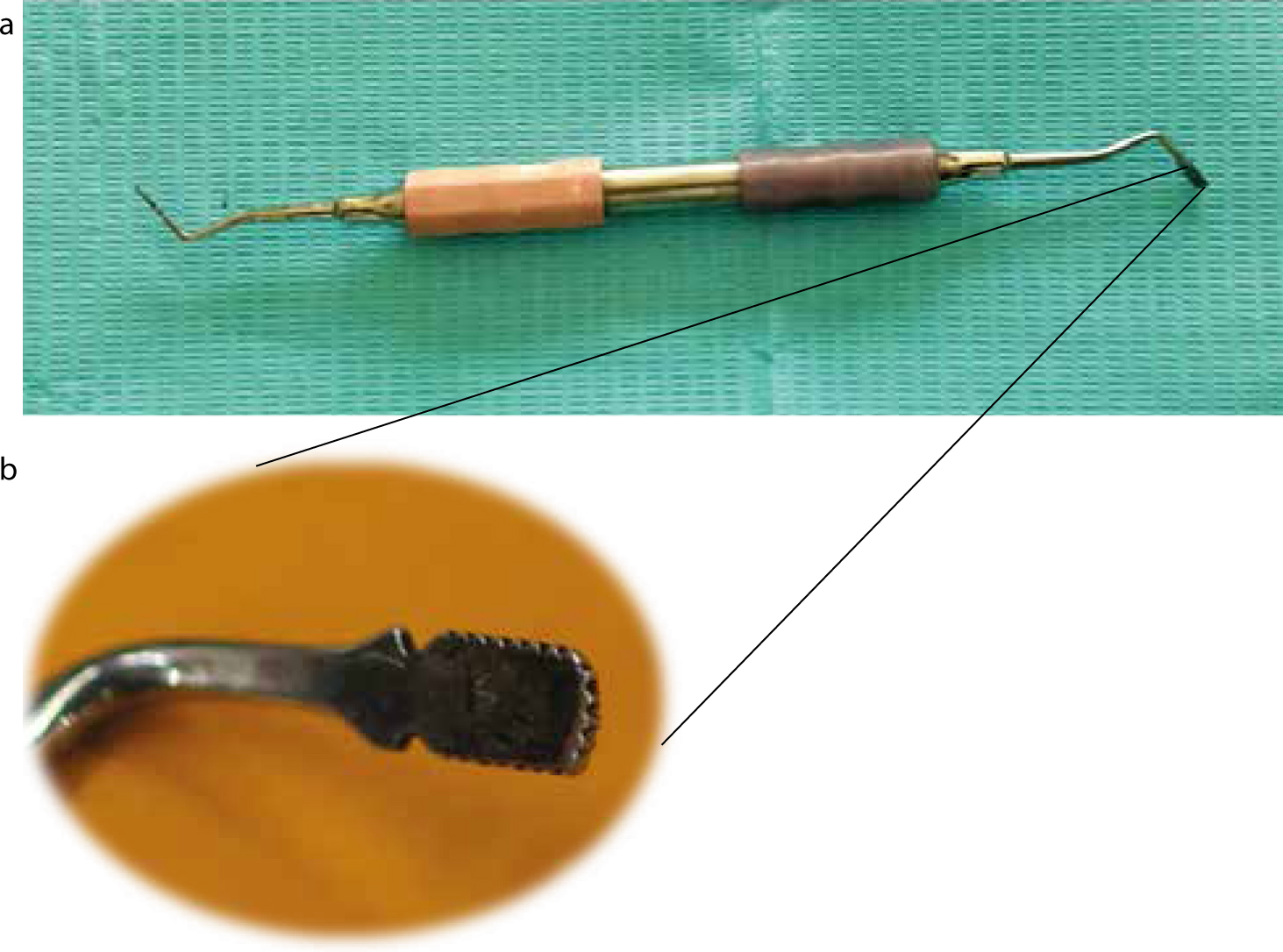
Cord packing technique
It is recommended that the packing of the retraction cord be initiated from the inter-proximal area. This can be done with the help of a periodontal probe and gentle pressure as the inter-proximal gingival is thin and delicate, with minimal depth of gingival sulcus. There are two broadly used techniques for packing retraction cord in the gingival sulcus depending on the clinical situation, the health of the gingival tissues, the depth of the gingival sulcus and the placement of the margin of the preparation on the tooth structure. A survey by Sorensen et al⁸ has shown that 98% of prosthodontists use cords out of which 48% use a dual cord technique and 44% use a single cord technique.
Single cord technique
This is a relatively straightforward method, usually employed for single teeth, with healthy gingival tissue. A single piece of retraction cord is packed into the gingival sulcus, followed by removal after adequate gingival displacement has been achieved. The impression of the tooth preparation margins can then be made.31 It is a useful technique when there is little or no haemorrhage from the gingival sulcus, and the preparation margins on the tooth are either gingival or slightly subgingival hydrated potassium aluminium sulfate.
Double cord technique
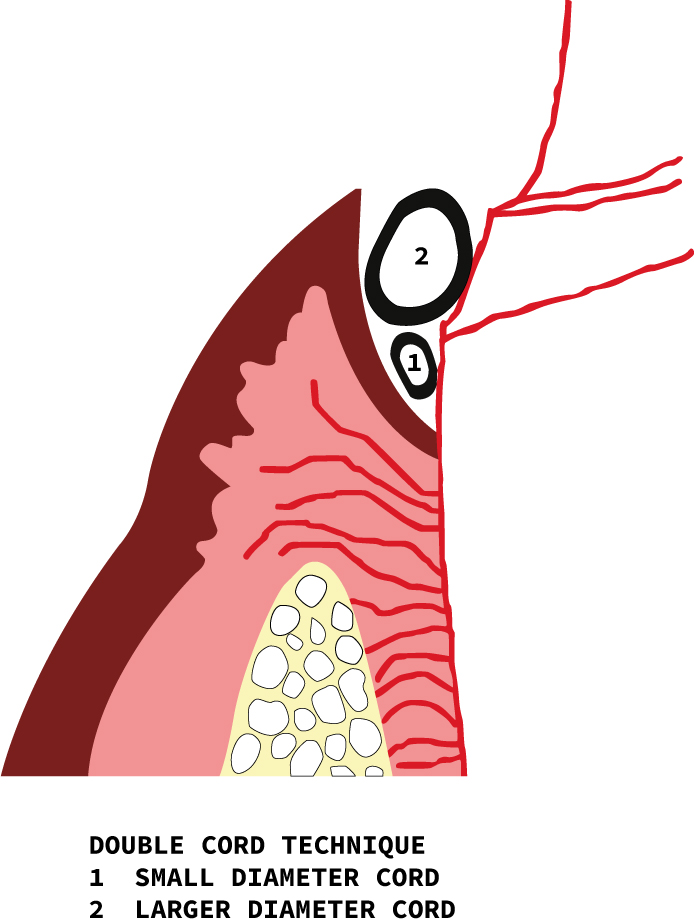
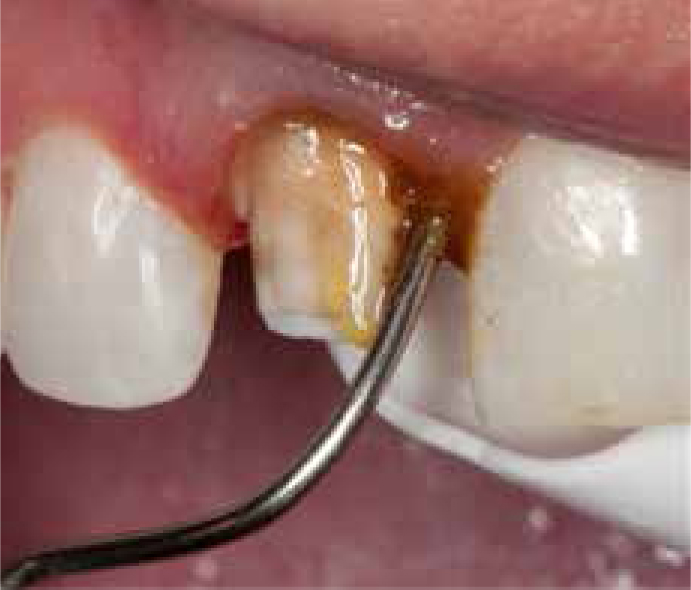
As the name indicates, two retraction cords are placed in the gingival sulcus, which is too deep to be sufficiently displaced with a single cord or where the tissue would collapse with the use of only a single cord. The margins of the tooth preparation in such cases may also be subgingival and hence require additional displacement of the gingival tissues. The technique describes placing a smaller diameter cord soaked with haemostatic agent into the depth of the sulcus, causing some lateral tissue displacement but primarily controlling haemorrhage. The second larger diameter cord is then packed into the sulcus, causing further lateral tissue displacement (Figure 7). The first deeper placed cord stays in place when the impression is made, after removal of the top, larger diameter cord.32 Care must be taken not to cause drying of the retraction cords, as they would then adhere to the gingival tissue and cause haemorrhage when removed.5
Cord positioning force
It is essential that non-damaging minimal force is utilized to insert the cord into the gingival sulcus, otherwise the displacement procedure can lead to haemorrhage and damage to the sulcular and junctional epithelium. Injudicious use of force during cord placement can lead to gingival recession later, due to disruption in blood supply and damage to the periodontal attachment fibres.9 There may be inadvertent excessive use of force while tucking the cord in the sulcus, particularly when the patient is anaesthetized.33 A study by Phatale et al34 has shown that the epithelial attachment sustains injuries at a force of 1 N/mm2, while it ruptures at 2.5 N/mm2, which is almost the same force required to place the retraction cord.
Cord retraction time
The time for which the cord is placed in the sulcus is also an important consideration. If the cord is placed for less than the recommended time, the gingival tissues may not be adequately displaced for the impression material to record the subgingival preparation margin. If the cord is placed for only two minutes, the sulcus width is reduced to 0.1 mm within 20 seconds of cord removal.5 On the other hand, if the retraction cord is placed for a longer time, this could result in damage to the gingival tissue and recession. This is especially relevant for pre-impregnated cords or cords used with haemostatic agents.35 Cords placed in the gingival sulcus for too long also have a chance of drying. If that happens, they adhere to the sulcular epithelium and tear the sulcular epithelium at the time of removal.36 The recommended time according to several studies ranges from 1–30 minutes.6,37,38 The goal should be to keep the cord in the sulcus for as little time as possible, so that any damage inflicted on the delicate soft tissues is minimized.39 This may be easier to do in the case of single tooth preparation but, for multiple teeth, it is important to keep a check on how long the cord has been in place for the tooth prepared earlier. The cord should be removed if excessive time is being taken to prepare subsequent teeth, and repacked once the procedure is complete.5 Also, the gingival sulci of all the prepared teeth should be checked after an impression has been made, so that no piece of cord is inadvertently left in the gingival sulcus.
Inspection of sulcus after retraction
After any gingival tissue retraction has been utilized, it is essential to wash and inspect the sulcus thoroughly for any adhering piece of retraction cord or residual impression material that may have broken off and be trapped in the sulcus. Washing also removes any chemicals or medicaments that may have been used in combination with the retraction cord. Any foreign body or filaments of retraction cords left in the gingival sulcus following the procedure can cause pain, swelling and increased inflammation as a result of foreign body reactions.40
The infusion technique
This technique uses a specially designed dento-infusor with a small tip containing a ferric sulfate medicament. The ferric sulfate medicament is available in two concentrations, 15% and 20% (Ultradent Products Inc, South Jordan, UT) with the 20% material being less acidic because of the presence of binders and coating agents and causing less removal of the smear layer from dentine. This formulation is also more viscous, which improves control during application.18 Before the impression is made, the brush-ended tip is used in a burnishing motion inside the sulcus gently extruding the medicament while encircling the tooth (Figure 8). If there are any isolated areas with persistent bleeding, the applicator tip can be used to exert firm pressure to that area for 2−4 seconds consistently, while extruding the medicament. Once haemostasis is achieved, a knitted retraction cord can be packed inside the gingival sulcus. When the impression is to be made, the cord is washed and removed, the sulcus rinsed with water and the impression made. The tissues can get reversibly discoloured when ferric sulfate is used and patients should be counselled beforehand accordingly. The discoloration usually dissipates in 24 to 48 hours.18
Cordless methods
Whenever a retraction cord is placed, there is some damage to the gingival tissue, as confirmed by histological studies.33,34 The damage is proportional to the force used to place the cord in the gingival sulcus.41 If the cord is packed into a sulcus where the gingival tissues are already damaged or inflamed, the inflammation may be exacerbated by the presence of the cord filaments.40 Studies have shown that the use of excessive force during placement of retraction cords results in greater chances of permanent damage to the periodontium, attachment loss and gingival recession.41 There was less tissue damage when a cordless retraction technique was used. Also, the presence of epinephrine in impregnated cord could result in tissue necrosis, when the cord is placed for longer than the recommended time.17 The cord packing procedure may also lead to bleeding and is uncomfortable to the patient, and hence local anaesthesia is frequently required.34
Materials used for the cordless retraction technique are available as pastes, foam or gel. They have the advantage of being non-traumatic to the gingival tissue during placement, leaving no residue, being easy to use and time saving. One study compared the pressure generated by retraction cords and cordless retraction techniques and found that cordless techniques put significantly less pressure (143 Kpa) on the gingival tissue as opposed to gingival retraction cords (5396 Kpa).42 Most products, however, have no haemostatic capability. Therefore, they may not be applicable in situations where there is laceration of gingival tissue, excessive haemorrhage or deep gingival sulcus.
Magic foam cord
This material is based on polyvinyl siloxane, with the ability to expand and displace tissues once placed inside the gingival sulcus. This is used in combination with a compression cap, which the patient bites on, followed by removal of the assembly and evaluation of the degree of retraction. If retraction is found to be satisfactory, the final impression can be made.
Expasyl
This is a viscous synthetic paste, which contains 10% aluminum chloride, 80% kaolin, with water and modifiers. The kaolin gives the material its physical properties and paste-like consistency, to help physically displace the gingival tissues while the aluminum chloride acts as a haemostatic agent, to control haemorrhage. The pressure exerted by the material when injected into the sulcus is considered non-damaging to the gingival tissues. It is available in capsules which are reusable and can be decontaminated. The small canula tip helps to insert the material into the sulcus. They are determined to be less painful to the patient during application, with quicker placement and less tissue damage,34 but the high concentration of aluminum chloride has been shown to be associated with tissue necrosis and sensitivity.33 The sulcus must also be thoroughly inspected to ensure that there is no residue of the retraction material, as aluminum chloride may inhibit the set of polyether impression materials.
Merocel
It is a synthetic polymer which is cut in 2 mm strips, and has a sponge-like texture. It is chemically extracted from hydroxylated polyvinyl acetate, which is a bio-compatible polymer. It has the ability to absorb fluid and, once placed in the gingival sulcus, swells and occupies the gingival sulcus (Figure 9). After removal, impression can be made revealing the finish line.40 It has many applications in ENT, gastric and otoneurosurgical procedures.43 Advantages include its ease of shaping and placement, being non-traumatic to gingival tissues, recovery of the tissue displacement within 24 hours and effective absorption of sulcular exudates.

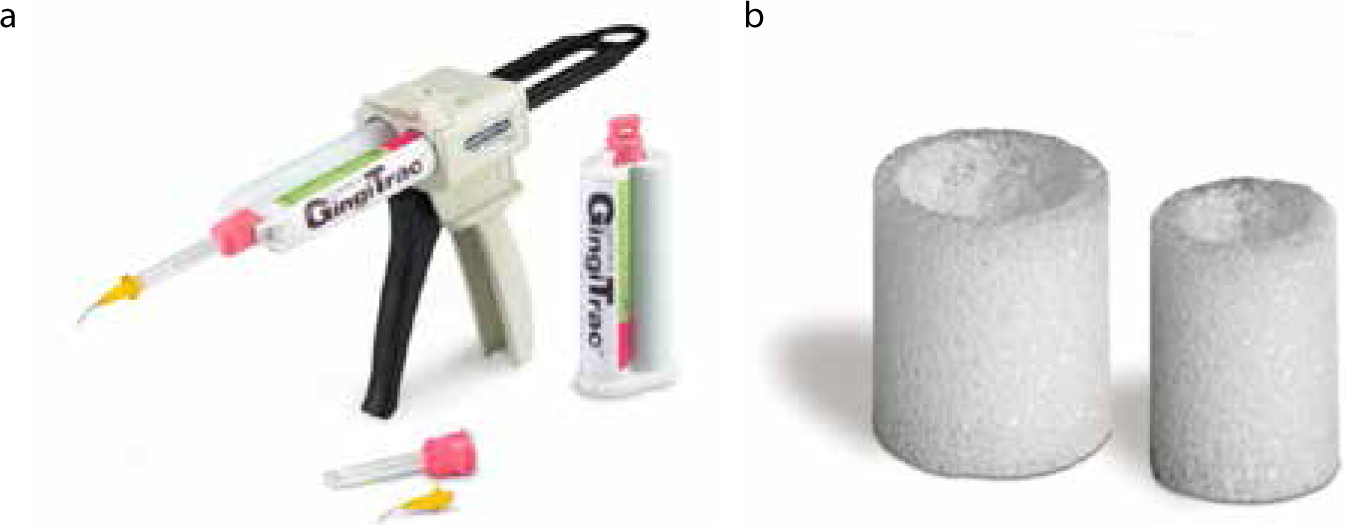
GingiTrac™
This product comes in combination with foamic cylinders to encircle the tooth. These cylinders are available in large and regular sizes. The technique involves the use of a polyvinyl siloxane paste to be inserted in the gingival sulcus (Figure 10). This is followed by placing the foamic cylinder filled with more of the retraction paste onto the tooth and directing the patient to exert biting pressure for 3−5 minutes, until the material sets. This is followed by removal of this assembly, and observation of the degree of retraction. If satisfactory, the final impression can be made, otherwise the procedure can be repeated. This is a relatively easy method with lesser trauma to the gingival tissue. Care must be taken not to use latex gloves when employing this product.
Retraction capsule
The astringent retraction paste is available as capsules which can be used with a composite capsule dispenser. The capsule has a long, slim nozzle with a soft edge, and allows the direct delivery of the high viscosity astringent paste containing 15% aluminum chloride, into the gingival sulcus. The nozzle also has an orientation ring marked in white, which corresponds to the size and position of the periodontal probe, and prevents excessive impingement of the delivery nozzle in the gingival sulcus (Figure 11).

Surgical methods
Some methods utilized to improve the visualization of the preparation margins of the tooth are not true retraction techniques. This is because they actually remove some part or all of the overlying gingival tissue in order to expose the finish line of the preparation and/or control haemorrhage. These techniques are more invasive and should only be used in cases where there is adequate amounts of attached gingiva. These methods include the following.
Rotary curettage
In this technique, a suitably shaped diamond bur (tapered fissure bur in most cases), is gently rotated around the gingival sulcus, slightly apical to the preparation margin, removing the lateral aspect of the gingival tissues. A retraction cord can then be placed in the trough created, to control haemorrhage and subsequently the impression can be made. A copious amount of water is needed when using this technique, and is only recommended for healthy gingival tissues. Case selection is important as removal of gingival tissue requires that there be adequate keratinized attached gingiva remaining.44 If keratinized tissue is not present, use of this technique results in gingival recession and deepening of the sulcus.45 The results of this technique are unpredictable, with increased chances of gingival recession in thin gingival biotypes. The tissue response has been shown to be comparable to that of electro-surgical tissue removal.46
Nonetheless, this technique should be used cautiously and, in cases where an aesthetic deficit is created, would not be readily visible.
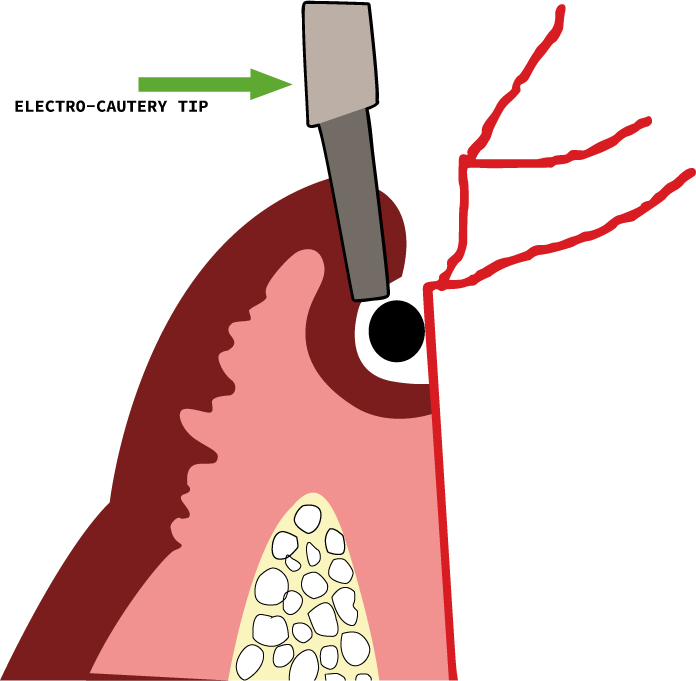
Electro-surgical tissue displacement
This technique is frequently employed in conjunction with retraction cords, especially in cases of gingival hyperplasia, excessive haemorrhage, deep subgingival preparation margins and to widen the gingival sulcus47 (Figure 12). Like all techniques for tissue removal, there are chances of excessive tissue removal followed by recession if not employed cautiously. The tip should be carefully placed so that the bone or cementum is not touched. If the electro-surgery electrode comes into contact with any metallic filling, adverse effects on the pulp and periodontium are observed.48 Also, this procedure is contra-indicated in patients with cardiac pace-makers and cardio-verter defibrillators, as the electromagnetic interference created by the electro-surgical units can detrimentally affect the working of the cardiac defibrillators.49 As far as the healing of the soft tissues after the use of electro-cautery is concerned, there was no significant difference between the wound healing when electro-surgery and scalpel were compared but, when used for deeper tissues or for longer periods of time, more damage and delayed healing was observed.50
Laser
Latest advances in dentistry have allowed the utilization of lasers for haemostasis and tissue removal. The soft tissue inside the gingival sulcus can be removed in order to visualize the preparation margins for an accurate impression. Although Diode lasers have been most commonly utilized for the purpose, Nd:YAG and Er:YAG lasers can also be used.5 There are studies indicating that gingival tissue displacement with lasers is less painful and can even be used without anaesthesia in selected cases.5 They result in minimal post-operative pain, haemorrhage and gingival recession.51 However, lasers run at higher operating cost and take more time to remove tissue than with electro-cautery or using a scalpel.52
Gingival retraction around implants
The soft tissue structures surrounding the natural teeth differ significantly from implants, in that the junctional epithelium around implants is less adherent, with increased permeability and decreased regenerative capacity53 (Figure 13). This results in an increased chance of damage and recession, when the peri-implant soft tissues experience any trauma resulting from retraction procedures, as compared to natural teeth.54Even after retraction, there is a greater tendency for peri-implant soft tissue to retract, as there is lack of support from the underlying peri-implant fibre structure, hence impressions are difficult to record, especially for deeply placed implants. A study comparing the various methods that could be utilized for the retraction peri-implant soft tissues, as compared to natural teeth, suggested that placement of retraction cords could result in more damage to the fragile supporting soft tissues adjacent to the implant.54 The use of chemicals, such as 15% aluminum chloride in an injectable kaolin matrix, is a better option, as it is minimally damaging to the junctional epithelium. However, its effectiveness is reduced with subgingival margins.55 In surgical options, lasers like Nd:YAG are contra-indicated for use near implants as their wavelength causes the implant to heat up and damage the surrounding bone.56 The Er:YAG laser can be used as it is reflected from metal surfaces but it is not as effective for haemostasis as CO2 laser.56 Electro-surgery is not recommended due to the risk of osseous necrosis and arcing through the metal implant. Rotary curettage should also not be attempted as lack of tactile control during removal of soft tissue can cause inadvertent damage to the surface of the implant. Lack of keratinized gingiva in the peri-implant area predisposes the tissues to recession if rotary curettage is attempted.54
Conclusion
Since gingival retraction is an integral part of clinical practice, the clinician should make an effort to utilize different methods and products available for retraction of gingival tissues in various clinical scenarios. Sometimes a combination of methods may be needed, and some things may work for one clinician and not for another. The effort put into the appropriate retraction of gingival tissues pays off in terms of longevity of restorations, better margins and aesthetics.

