Abstract
Ebola is a highly dangerous infectious disease seen mainly in West Africa or travellers from there. All healthcare workers should check the recent travel history of their patients and follow formal guidance issued.
From Volume 42, Issue 1, January 2015 | Pages 7-21
Ebola is a highly dangerous infectious disease seen mainly in West Africa or travellers from there. All healthcare workers should check the recent travel history of their patients and follow formal guidance issued.

Ebola is the common term used for Ebola Virus Disease (EVD) – just one of the lethal Viral Haemorrhagic Fevers (VHFs) seen mainly in Africa. Ebola is a classic disease which can be transmitted to humans from animals (a ‘zoonosis’) with persistence of the virus in reservoir species, such as fruit bats, found in endemic areas. Ebola can cause disease both in humans and non-human primates.
Ebola is not new; it was first identified in 1976 in sub-Saharan Africa. Typically fewer than 500 cases have been reported annually, usually in rural areas, and between 1979 and 1994 there were no cases reported. The Ebola outbreak in West Africa, first reported in March 2014, rapidly became the deadliest outbreak of Ebola.
The current virulence of Ebola, epidemic spread in West Africa including in urban conurbations, and sporadic appearances in many geographic locales including North America, Europe and the Antipodes, mainly through infected travellers and healthcare workers, raises grave concerns of an impending pandemic. Although by far most common in the resource-poor west African countries, with the continually increasing global travel, Ebola is appearing or may appear in most countries globally.
Human-to-human transmission of Ebola is known only through direct contact with the tissues, blood, secretions, or other bodily fluids, including saliva, of infected people or animals, and with environments contaminated with such fluids. Infections in healthcare settings have generally been due to healthcare workers treating patients with suspected or confirmed Ebola, when infection control precautions were not strictly and effectively practised.
To date there have been no reported cases of transmission of Ebola in dental settings. However, the fact that the virus may be transmitted through human blood and secretions, including saliva, and that the incubation period could last up to 21 days, implies that oral healthcare workers (mainly in the endemic areas) may run the risk of acquiring Ebola if meticulous infection control measures are not routinely followed. In addition, the fact that there are potentially contagious asymptomatic or mildly symptomatic individuals infected with Ebola who could seek oral or dental care suggests that oral and dental healthcare providers must be at potential risk of infection.
Accurate history including questioning about any travel in the previous month, careful patient examination and the routine, scrupulous use of infection control measures are probably enough to minimize such a risk. Patients who may be infected may have elective dental care deferred for 3–4 weeks (at least 21 days).
There are many other health and other issues; for example, the Ebola outbreak has also spawned considerable fear, and cases of malaria, pneumonia and typhoid have gone untreated because people in those countries hit hardest by Ebola cannot find a clinic for them or are too afraid to go to one.
The Viral Haemorrhagic Fevers (VHFs) are a diverse group of human and animal illnesses characterized by fever, bleeding disorders and often progression to shock and death. Some – notably Ebola, Lassa and Marburg fevers – are potentially highly lethal and these are found especially in sub-Saharan Africa (Table 1). Some VHF agents, however, including some Hantaviruses (first recognized in Korea), are encountered worldwide including in western countries, and humans often become infected through contact with rodent urine, saliva, or faeces. Hantaviruses may also cause potentially lethal renal or pulmonary disease but they may also cause relatively mild illnesses. More than 20 hantaviruses are recognized.
| Families | VHFs Resulting |
|---|---|
| Arenaviridae | Cause: |
| Bunyaviridae | Hantavirus genus – causes haemorrhagic fever with renal syndrome (HFRS) |
| Filoviridae | Ebola virus Marburg virus |
| Flaviviridae | Dengue, yellow fever, and two viruses in the tick-borne encephalitis group – Omsk haemorrhagic fever virus and Kyasanur Forest disease virus |
The diverse spectrum of distinct RNA virus families that cause VHFs and their human manifestations, shown in Table 1, share a number of features:
The viruses that cause VHFs are distributed over much of the globe but, since each virus is associated with one or more particular host species, the diseases are usually endemic mainly or only where the host species live(s). While people usually become infected only in areas where the host lives, occasionally they become infected by a host that has been exported from its native habitat. Because increasingly more people travel each year, outbreaks of these diseases are appearing in places where they rarely, if ever, have been previously encountered and, of course, people are increasingly travelling to endemic areas for vacations and business. The current, albeit uncommon, spread of Ebola to parts of USA, Europe, the Antipodes and elsewhere is a good example where the disease has spread from its endemic sub-Saharan habitat.
Yellow fever is one of the most commonly known VHFs, endemic in both South America and Africa, mosquito-borne, but a vaccine is available. Another common mosquito-borne VHF endemic to Latin America and Asia is dengue fever. The latter VHF has recently reached epidemic proportions in some regions of South-East Asia and the Indian sub-continent. In Asia and Europe – where most VHFs are caused by hantaviruses (Bunyaviridiae) spread by rodents, the dangerous haemorrhagic fever with renal syndrome (HFRS) may be seen. The VHFs also include Hantaan River virus, Puumala virus and Seoul virus, all spread to humans by rodents (typically mice), via faeces, urine or airborne. A few host reservoirs, such as the common rat, are found worldwide and can carry Seoul virus; therefore, anywhere where the common rat is found, humans can contract VHFs.
In North and South America, the VHF hantaviruses cause severe respiratory infections, or pneumonia – hantavirus pulmonary syndrome (HPS). In the USA and Canada, most HPS is caused by the Sin Nombre hantavirus. However, there are no reports of person-to-person transmission of hantaviruses in North America. There are also many hantaviruses that can cause HPS in South America.
In Africa, bats of the Pteropodidae family are the natural reservoir for the similar Marburg virus and Ebola. These two viruses are both Filoviruses and can spread among humans when a person comes into contact with the bodily fluids of an infected person, or if an infected animal is eaten – but not by air. There are many species of animals that serve as natural reservoirs for viruses that cause VHFs. For example, Ebola Zaire, the strain of Ebola causing the current outbreak, is believed to have been transferred to humans by fruit bats.
Another VHF, the Lassa virus (an Arenavirus), also found in Africa in a reservoir rodent the multi-mammate rat, can be transmitted when rat faeces or urine containing virus become airborne, or the infected animal is eaten. Thus it is evident that some VHFs can be transmitted to humans by the airborne route. Although VHFs are principally transmitted to humans by mammals, and by contact, Crimean-Congo haemorrhagic fever can be spread to people through the bites of ticks infected with the Bunyavirus that causes this VHF. These ticks can also infect livestock, such as cattle, sheep and goats or birds – usually ostriches.
Ebola viruses are lipid-enveloped, non-segmented, negative-stranded RNA viruses belonging to the genus Ebolavirus in the family Filoviridae. Filoviruses are 80 nm in diameter twisted filaments (hence the name) up to 1.1 µm long (Figure 1).
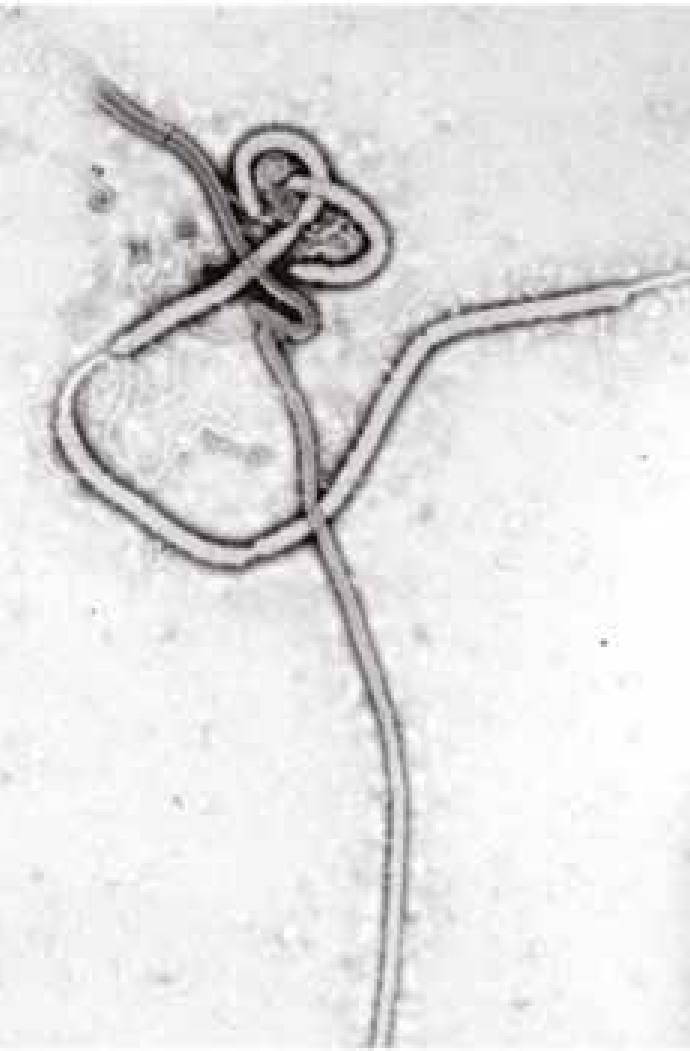
Five different Ebola virus species are recognized, namely Bundibugyo, Zaire, Sudan, Reston and Taï Forest. Humans and other primates are susceptible to infection, and regarded as end hosts and not as reservoir species. The three first species (Bundibugyo, Zaire, Sudan) have caused epidemics in Africa with high case-fatality rates. No human deaths due to the two other Ebola species have been reported.1
Ebola is dependent on an animal natural reservoir, mainly fruit bats and non-human primates, often eaten by humans as ‘bush meat’. In an outbreak, the human index cases usually become infected after contact with an infected animal or its blood, in most cases during hunting. Secondary human cases become infected after close contact with another human case, with infected human fluids or tissues, or with a recent dead corpse of a human case. Ebola viruses are easily transmitted by direct contact or by contact with patient body fluids, mainly blood. As such, health professionals working under sub-optimal conditions usually constitute a large proportion of Ebola victims.2 Human-to-human transmission is due to exposure to blood and, possibly, other bodily fluids of an infected individual or animal, through abrasions in the skin, mucosal lesions or through parenteral transmission.3 Transmission is most likely in later stages of disease.
Ebola causes a typical acute haemorrhagic fever, characterized by a high lethality rate. Humans, if infected, have the virus in tissues, bodily fluids including saliva, and in all body orifices.
The Ebola genome encodes eight proteins, four of which counteract the host immune responses:
Ebola thus disables the immune system dendritic cells and then via a cytokine storm disrupts the vascular system, culminating in widespread haemorrhage, hypotension, followed in some by shock and death. The infected dendritic cells are unable to mount an interferon response, macrophages are impeded, inflammatory proteins and nitric oxide released damaging blood vessels and endothelial cells leading to blood leakage.5 Ebola also damages the liver (dysregulating blood coagulation proteins), the adrenal glands and blood pressure regulation (leading to circulatory failure), and the gastro-intestinal tract (leading to diarrhoea).
Ebola incubation can be up to 21 days (often 3–8). Early signs and symptoms are often non-specific, such as fever, fatigue, myalgia and malaise. In Ebola, there is often a sudden onset of high fever, chills, headache, myalgia, anorexia, nausea, abdominal pain, sore throat and prostration. Liver damage may cause DIC (disseminated intravascular coagulopathy), and bone marrow dysfunction, and the patient may bleed under the skin, in internal organs, or from the mouth or other body orifices. In some cases, an exanthematous rash appears on the trunk with widespread haemorrhagic manifestations, conjunctivitis, haematemesis, melaena, epistaxis and haematuria.6,7,8 The key features of Ebola (alphabetically arranged) can be summarized as follows:
*Major features: these can appear suddenly, between 2 and 21 days after infection, usually after 5–7 days.
In one study, 89% of the patients had fever, 80% had headache, 66% felt weak, 60% were dizzy, 51% had diarrhoea, 40% had abdominal pain and 34% had vomiting – but only one patient had bleeding. Severely ill patients may develop shock, delirium, seizures and coma (Table 2).
| Days | Phase | Main features | Other features |
|---|---|---|---|
| 0–3 | Early febrile | Fever | Malaise, fatigue, body aches |
| 3–10 | Gastro-intestinal | Epigastric pain, nausea, vomiting, diarrhoea | Persistent fever, headache, conjunctival injection, chest and abdominal pain, arthralgia, myalgia, hiccups, delirium |
| 7–12 | Shock or recovery | Shock: diminished consciousness or coma, rapid thready pulse, oliguria, anuria, tachypnoea | Recovery: resolution of gastro-intestinal symptoms, increased appetite, increased energy |
| ≥10 days | Late complications | Gastro-intestinal haemorrhage | Secondary infections, oral or oesophageal candidiasis, persistent neurocognitive abnormalities |
A clinical Ebola diagnosis is usually confirmed by blood testing (Tables 2 and 3: http://www.cdc.gov/vhf/ebola/hcp/interim-guidance-specimen-collection-submission-patients-suspected-infection-ebola.html). Whole blood preserved with EDTA is preferred. Ebola virus is detected in blood only after the onset of symptoms, usually fever. Virus is generally detectable by real-time RT-PCR from 3–10 days after symptoms appear. Specimens ideally should thus be taken when a symptomatic patient first reports to a healthcare facility and is suspected of having Ebola exposure. However, if the onset of symptoms is <3 days, a further specimen may be needed to rule-out Ebola virus completely, if the first specimen tests negative.
| Classificaton Criteria | |
|---|---|
| Suspected | Any person, alive or dead, who has (or had) sudden onset of high fever and had contact with a suspected, probable or confirmed EVD case, or a dead or sick animal OR any person with sudden onset of high fever and at least three of the following symptoms: headache, vomiting, anorexia/loss of appetite, diarrhoea, lethargy, stomach pain, aching muscles or joints, difficulty swallowing, breathing difficulties, or hiccup; or any person with unexplained bleeding OR any sudden, unexplained death. |
| Probable | Any suspected case evaluated by a clinician OR any person who died from ‘suspected’ EVD and had an epidemiological link to a confirmed case but was not tested and did not have laboratory confirmation of the disease. |
| Confirmed | A probable or suspected case is classified as confirmed when a sample from that person tests positive for EVD in the laboratory. |
All healthcare and laboratory personnel collecting or handling potentially infectious materials specimens must follow established standards: this includes blood and saliva. These standards include wearing appropriate Personal Protective Equipment (PPE) and following all safety rules for all specimens regardless of whether they are identified as being infectious.
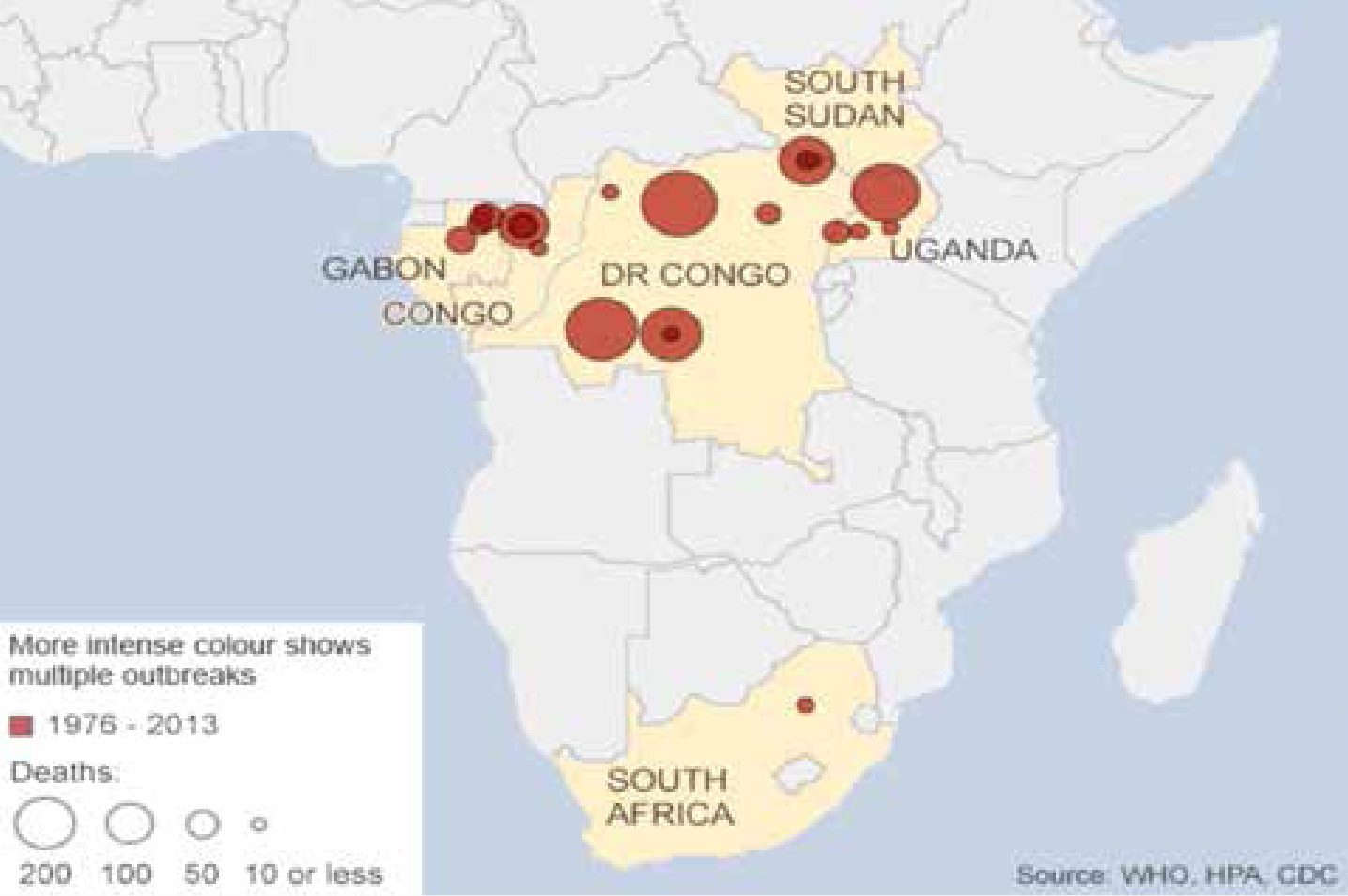
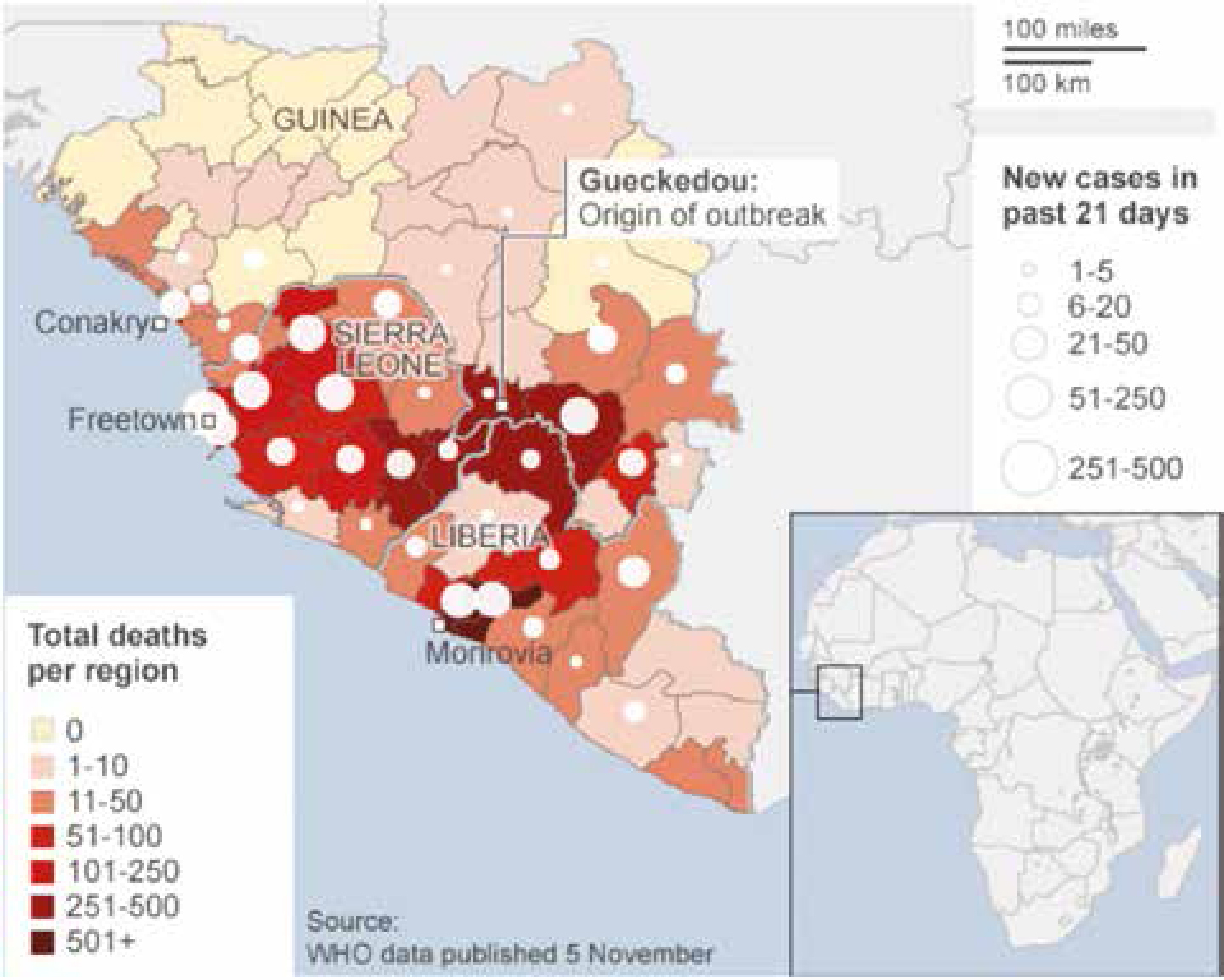
Ebola is not new but is a potentially lethal Viral Haemorrhagic Fever (VHF) that has been smouldering in sub-Saharan and West Africa at least since the 1970s – possibly unrecognized for many decades (Figures 2–9 and Table 4: adapted from WHO). The first cases of Ebola recognized were in 1976 between Zaire (now Democratic Republic of the Congo: DR Congo) and Sudan (now Republic of South Sudan), and were named Ebola after a north-western Zaire river.

| Initial Year of Outbreaks | Main Countries Affected | Reported Cases | Reported Deaths |
|---|---|---|---|
| 1976 | DR Congo | 318 | 280 |
| 1976 | Sudan | 284 | 151 |
| 1994 | Gabon | 52 | 31 |
| 1995 | DR Congo | 325 | 254 |
| 1996 | Gabon | 60 | 45 |
| 2000 | Uganda | 425 | 224 |
| 2001 | Gabon | 65 | 53 |
| 2001 | Congo | 59 | 44 |
| 2003 | Congo | 143 | 128 |
| 2007 | DR Congo | 264 | 187 |
| 2007 | Uganda | 149 | 37 |
| 2012 | DR Congo | 57 | 29 |
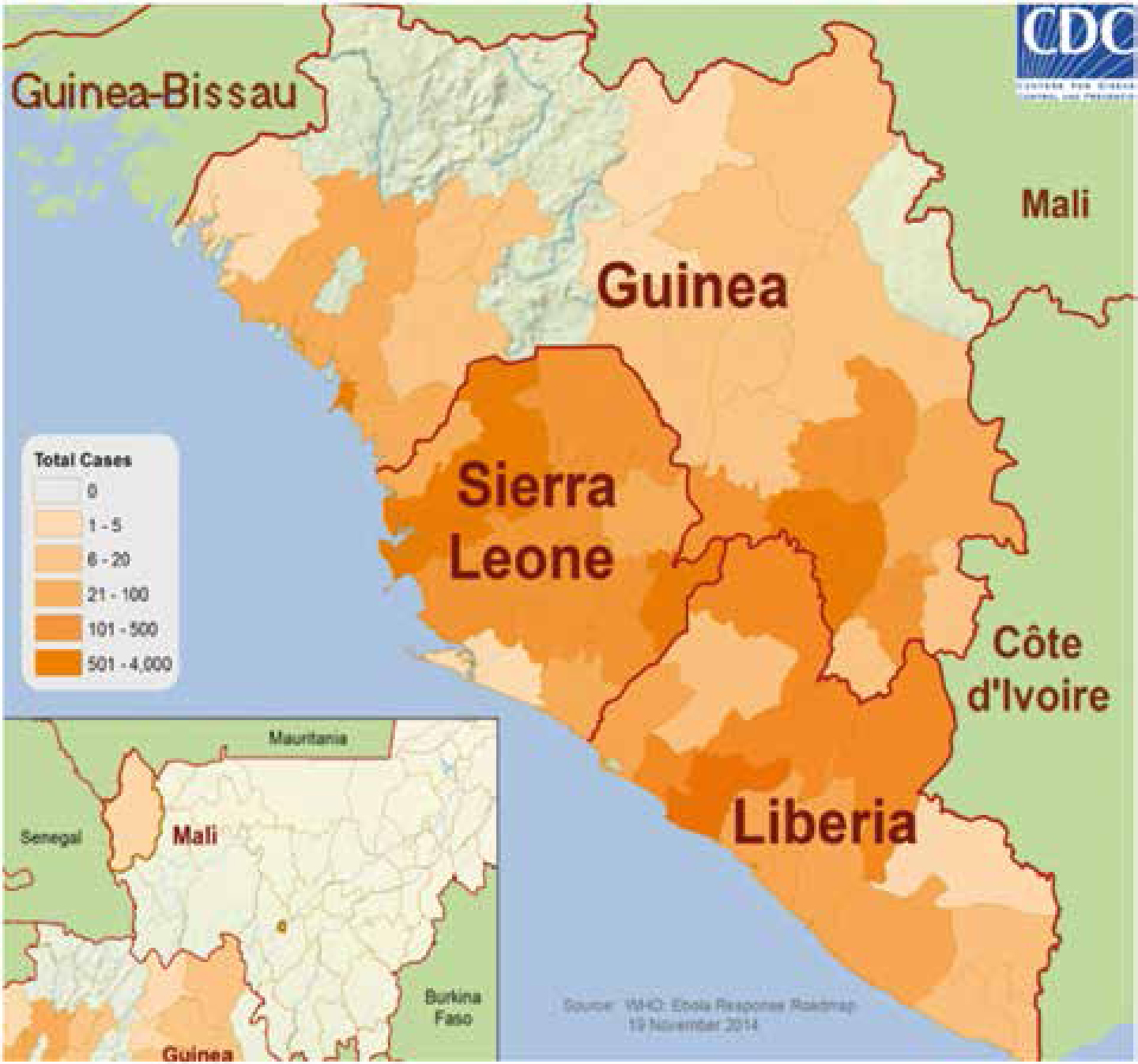
| 2014 | Guinea, Liberia, Sierra Leone | 15000+ | 5700+ |
| Countries with widespread transmission | Affected areas |
| Guinea | Entire country |
| Liberia | Entire country |
| Sierra Leone | Entire country |
| Countries with an initial case or cases and/or localized transmission | Affected areas |
| DR Congo | Entire country |
| Mali | Kayes, Kourémalé, Bamako |
| United States | Dallas (Texas), New York City |
| Previously affected countries | Affected areas |
| Nigeria | Lagos, Port Harcourt |
| Senegal | Dakar |
The first 1976 Ebola outbreaks were in DR Congo and Sudan, typically in rural settings (Table 4). About 6% of healthy controls living and/or working in the same areas had high serum antibody levels against Sudan Ebola virus11 and this figure for the Zaire Ebola virus was 1%, confirming that replicative Ebola infection may occur in asymptomatic individuals.12 Therefore, human-to-human transmission is obviously possible involving both ill and asymptomatic carriers, though is much more likely in ill people. Overall, the Ebola case-fatality rate, estimated on the basis of outbreaks occurring between 1976 and 2012, was 65% but, with a level at 95%, the Ebola Zaire species seems the most lethal.13 In general, all haemorrhagic cases have had a fatal outcome within about a week.
The current Ebola epidemic is caused by Ebola (Zaire species), unlike many of the previous epidemics. It first appeared in a two-year-old child, who died on 6 December 2013 in Meliandou, a small village in the Gueckedou region of south-eastern Guinea. In March 2014, Guinea hospital staff alerted their Ministry of Health and the charity Medecins Sans Frontieres (MSF) to an unusual disease in the south-eastern regions of Gueckedou, Macenta, Nzerekore and Kissidougou, which caused fever, diarrhoea and vomiting and had a high death rate. Of the first 86 cases recognized, 59 died. The World Health Organization (WHO) later confirmed the disease as Ebola.
On 23 March 2014, the WHO was notified of the outbreak in Guinea and, by the end of March, Ebola had crossed into Liberia and was then confirmed in Sierra Leone in May. In June, MSF described the Ebola outbreak as ‘out of control’. Nigeria had its first case of Ebola in July and, in the same month, two leading doctors died from Ebola in Liberia and Sierra Leone. By 8 August, the WHO had declared the epidemic to be a ‘public health emergency of international concern’14 and Senegal reported its first Ebola case on 29 August.
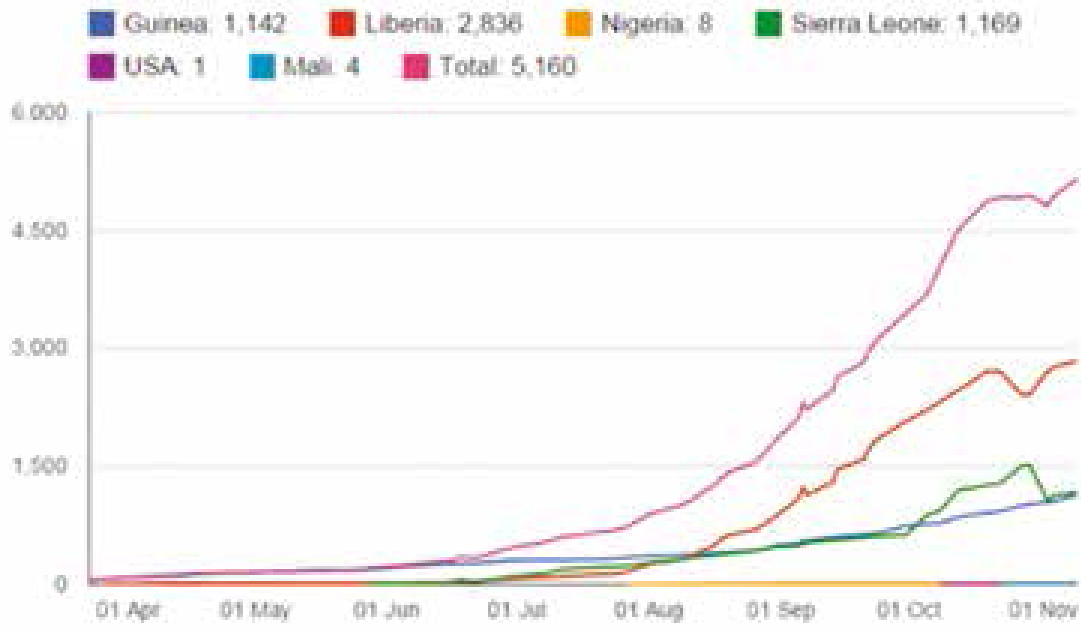
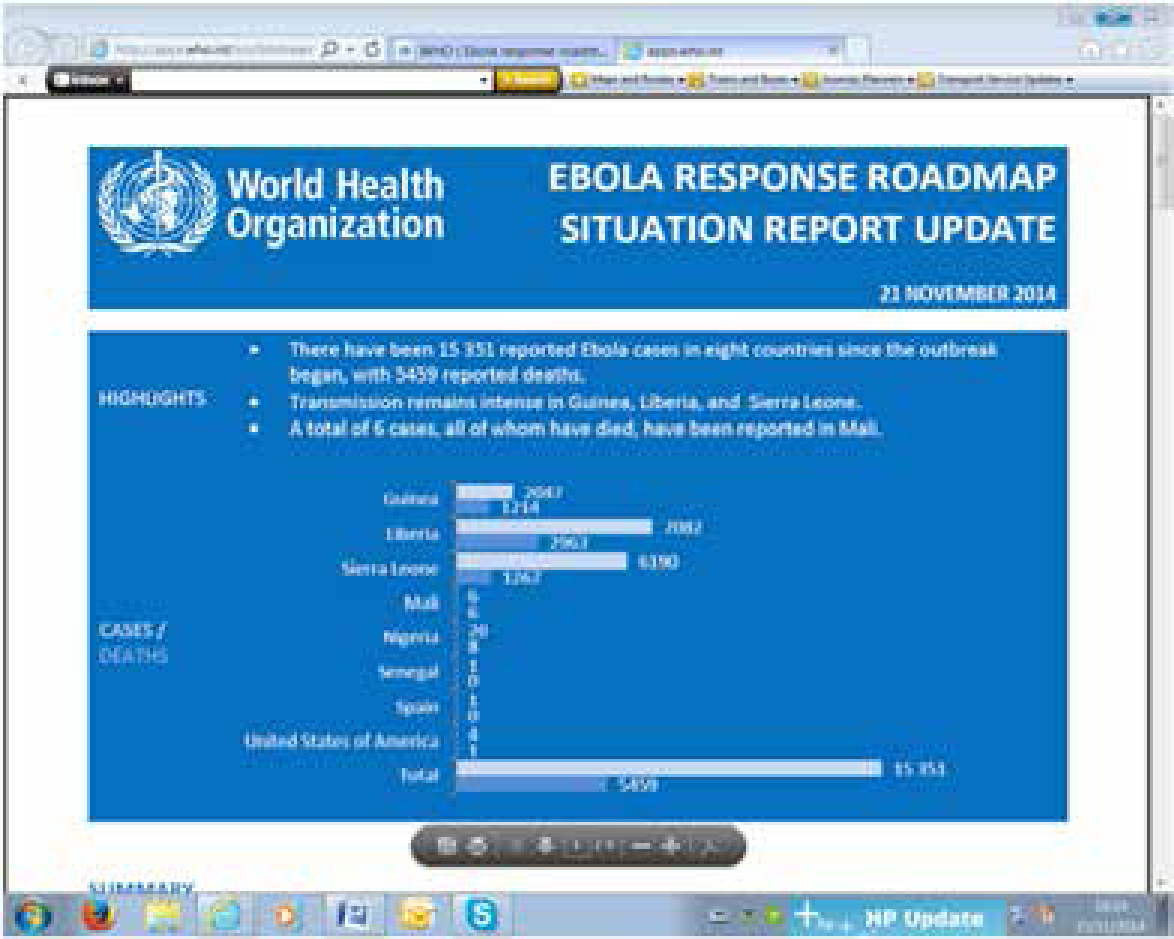
Ebola outbreaks in earlier years had primarily been in remote villages but the current epidemic has affected virtually these entire West African countries, including major urban centres, and capital cities. By 21 November, WHO had reported 15,351 Ebola cases in eight countries since the outbreak began, with 5,459 reported deaths. A total of six cases, all of whom have died, have also been reported in Mali. By December 2014, more than 15,500 probable and confirmed cases had been reported, mainly from five west African countries – Guinea, Liberia, Nigeria, Senegal and Sierra Leone (Ebola Response Team, 2014) – more than all previous Ebola epidemics combined, and is of serious concern because of high death rates up to 70%. The WHO admits the figures are underestimates given the difficulty collecting the data.
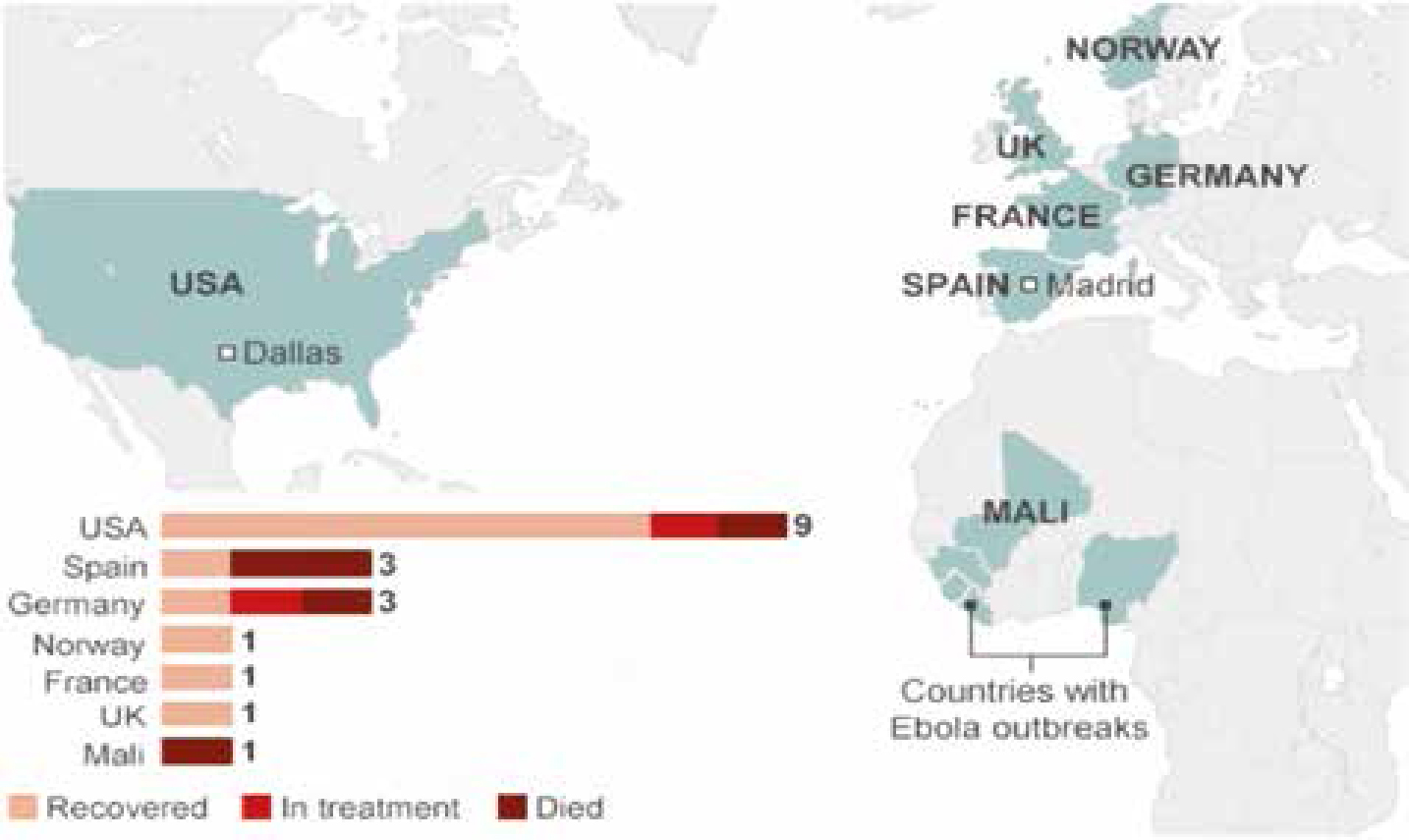
Infection outside Africa has been mainly restricted to healthcare workers infected with Ebola while in West Africa (Figure 3). The first case of Ebola on US soil was Thomas Eric Duncan, who contracted Ebola in Liberia before travelling to the US, displaying symptoms of disease by 24 September in Dallas, Texas, five days after his arrival and dying on 8 October. Two medical workers in Dallas who treated Duncan tested positive for Ebola since his death.
Teresa Romero, the first person to contract Ebola outside West Africa, was a Spanish nurse infected after looking after two missionaries infected with Ebola who had returned from Liberia and Sierra Leone to Spain. These infections of healthcare workers have been attributed to failed infection control procedures. The number of healthcare workers infected with Ebola remains a concern. There had been 546 infections and 310 deaths of healthcare workers by November 2014. Germany, Norway, France and the UK have all treated patients who contracted Ebola in West Africa and there have been ‘scares’ elsewhere, such as in Macedonia and Australia.
The WHO has declared the outbreaks in Nigeria and Senegal officially over, as there have been no new cases reported since 5 September. MSF confirmed that for the number of new Ebola cases in Liberia, the worst-hit country, two-thirds of the 696 beds in the country's treatment centres were empty in November. However, MSF warned that the disease could ‘flare up’ again, such as in Guinea, where the case numbers are rising again, and Sierra Leone, where cases are also increasing in number.
A separate outbreak of Ebola virus infections, unrelated to this West African outbreak, has killed 49 people in the DR Congo but had, by the end of November, been declared over.
Host-to-human transmission of Ebola is only through direct contact with, or consumption of the tissues, blood, secretions, or other bodily fluids, of infected hosts such as non-human primates or fruit bats (in ‘bush meat’), and with environments contaminated with such fluids. These products appear outwith the continent of Africa often transported illegally in personal luggage. There is no evidence of airborne spread of Ebola.
Human-to-human transmission of Ebola is only through direct contact with the tissues, blood, secretions, or other bodily fluids, including saliva, of infected hosts, and with environments contaminated with such fluids. Infections in healthcare settings have been due to healthcare workers treating patients with suspected or confirmed Ebola when infection control precautions were not strictly practised.
Prevention efforts to prevent transmission of VHFs concentrate on avoiding contact with patients with acute infection, as well as the disease-harbouring host species. If prevention methods fail and a case does occur, efforts should focus on preventing further transmission from person to person. Because many of the hosts that carry VHF are rodents, disease prevention efforts include:
For VHF spread by arthropod vectors, prevention often focuses on community-wide insect and arthropod control. In addition, people are encouraged to use insect repellants, proper clothing, bed nets, window screens and other insect barriers to avoid being bitten.
For VHFs such as Ebola that can be transmitted from one person to another, avoiding close physical contact with infected people and their body fluids is the most important way of controlling the spread of disease. Barrier nursing or infection control techniques include isolating infected individuals and wearing protective clothing. Other infection control recommendations include proper use, disinfection, and disposal of instruments and equipment used in treating or caring for patients with VHFs. Care with sharps is essential. Environmental decontamination is typically accomplished with phenolics or hypochlorite (eg bleach). Household bleach or an EPA-registered hospital disinfectant will kill Ebola. Ebola virus is readily destroyed even by washing hands with soap and water.
The CDC has developed practical, hospital-based guidelines, entitled Infection Control for Viral Haemorrhagic Fevers in the African Health Care Setting (http://www.cdc.gov/vhf/abroad/vhf-manual.html (last accessed, November 22, 2014). The CDC has also made clear the following issues with regard to Ebola transmissibility (adapted from guidelines: http://www.cdc.gov/vhf/ebola/pdf/top-10-things pdf):
CDC has been widely criticized for projecting overconfidence in US hospitals' capacity to manage Ebola. Health forecasters faced a trade-off: communicating uncertainty often undermines perceived expertise, but if they do not communicate uncertainty and end up being wrong, they risk losing even more credibility. Unfortunately, any new health threat comes with uncertainties.15
http://www.nbcnews.com/feature/making-a-difference/boston-doctor-returns-sierra-leone-fight-ebola-n247411
Checklists for evaluating Ebola risks are shown in Figure 9.
Healthcare workers are extremely susceptible to catching Ebola from infected patients. Infection in healthcare settings has been due to healthcare workers treating patients with suspected or confirmed Ebola, especially when infection control precautions were poor.
Ebola transmission through the air has not been documented in the natural environment. However, there is one report in experimental monkeys of fatal aerosol transmission of Ebola virus, which reinforces the importance of taking appropriate precautions to prevent any potential aerosol transmission to humans.16 CDC recommendations include wearing appropriate personal protective equipment (PPE) and specify that respirators, such as an N95 respirator, or a powered air-purifying respirator should be worn when managing Ebola-infected patients (http://www.cdc.gov/media/releases/2014/fs1020-ebola-personal-protective-equipment.html, accessed 22 November 2014).
An immediate quarantine and thorough examination should be performed on suspected cases to rule out Ebola, while also ensuring appropriate PPE is readily available and properly utilized. The risk of infection increases as the extent and the frequency of contact increase.17 A lethal viral dose, via a needlestick exposure from an acute-phase patient, has been reported.18 There are no reports of transmission through saliva contamination though saliva may be infective.
Extreme care must be taken with infected blood, secretions, excretions, tissues, hospital materials and waste. Reports indicate that those who recovered from the disease could transmit the virus for up to two months after recovery at least through their semen.
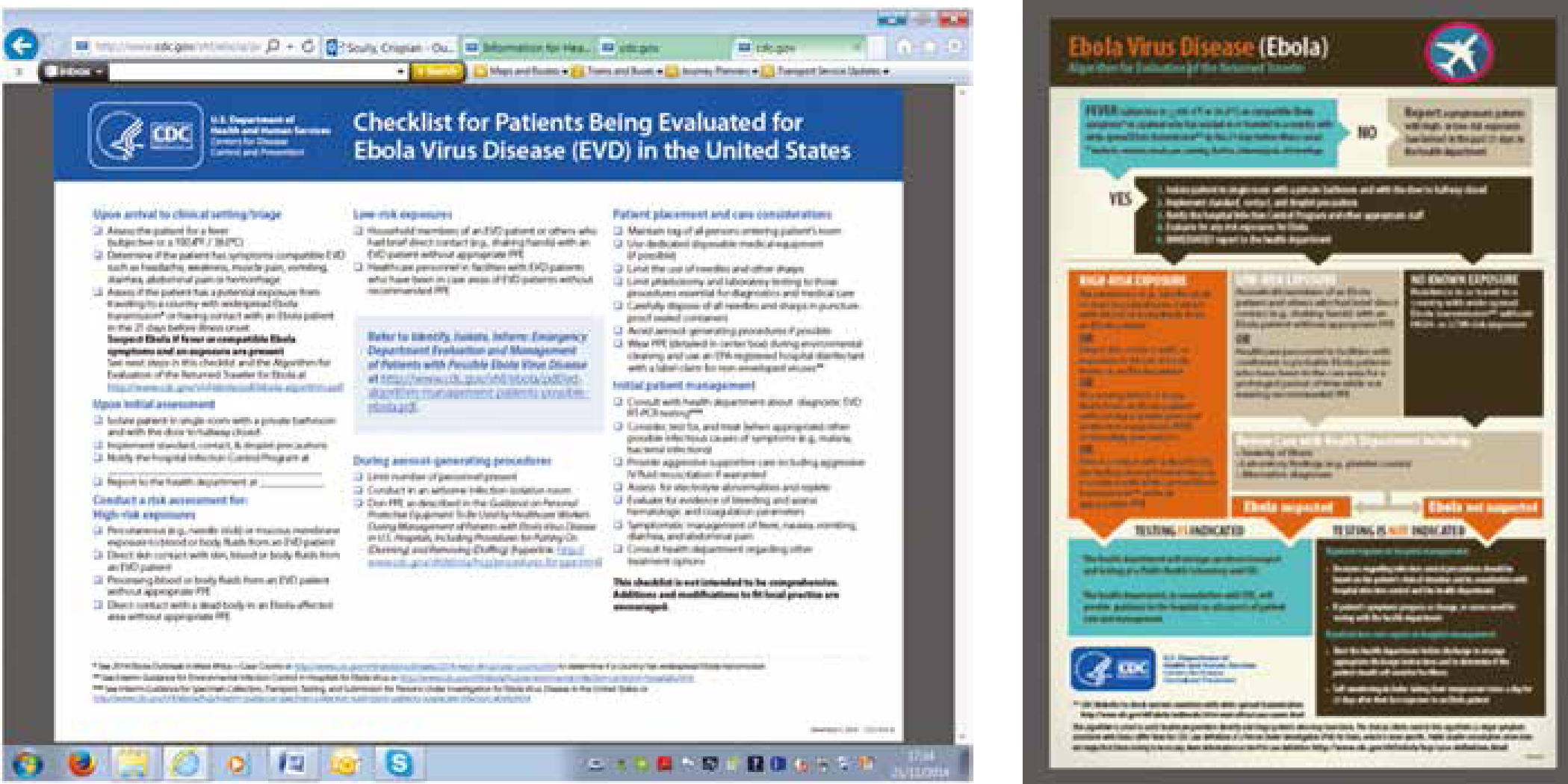
CDC has recently issued a checklist for patients being evaluated for Ebola (http://www.cdc.gov/vhf/ebola/pdf/checklist-patients-evaluated-us-evd.pdf). The essentials (Table 6) are to:
| Ebola Risk | Criteria |
|---|---|
| High | Having lived in the immediate household and provided direct care to a person with Ebola while the person was symptomatic. |
| Some | Close contact in households, healthcare facilities, or community settings with a person with Ebola while the person was symptomatic. |
| Low | Having been in a country with widespread Ebola transmission within the past 21 days and having had no known exposures. |
| None | Contact with an asymptomatic person who had contact with person with Ebola. |
US and various other government authorities have issued travel restriction warnings to Ebola-affected areas. If travel is essential then the following guidelines should be followed, although this is not an exhaustive list:
If you must travel to an area affected by the 2014 Ebola outbreak, CDC recommends (http://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/qa.html):
Prevention efforts concentrate on avoiding contact with patients with acute infection. The viruses are readily destroyed by washing hands with soap and water. Barrier nursing or infection control techniques include isolating infected individuals and wearing protective clothing. Other infection control recommendations include proper use, disinfection, and disposal of contaminated instruments and equipment. Environmental decontamination is typically accomplished with hypochlorite (eg bleach) or phenolics. With these measures, Ebola has been largely contained in Senegal and Nigeria.
Quarantine was introduced in USA but WHO rejects this idea and, after being evaluated in co-ordination with the CDC and her treating clinicians in Newark, the first quarantined US patient was discharged (27 October). This is a rapidly changing scenario. The Center for Disease Control and Prevention (CDC) in the USA has developed guidelines, entitled Infection Control for Viral Haemorrhagic Fevers in the African Health Care Setting. Available at http://www.cdc.gov/vhf/abroad/vhf-manual.html.
It is unlikely that dental healthcare workers may treat many individuals with manifest Ebola, owing to the severity at advanced stages. However, it is believable that asymptomatic patients and those with mild forms, who are unaware of their status, may seek or undergo dental therapy.
Individuals coming from endemic areas, particularly during outbreaks, could be carriers, whether asymptomatic or mildly symptomatic, and should initially be managed as if they could be contagious. Clinical examination may help oral and dental healthcare workers recognize or suspect individuals with mild or moderately severe forms of Ebola who might seek oral healthcare. Orofacial manifestations recorded are listed in Table 7.
| Site | Signs and Symptoms | ||
|---|---|---|---|
| General | Fever | Malaise | Diarrhoea and vomiting Musculoskeletal pain Others |
| Skin | Injection site bleeding | Rash | |
| Ocular | Bleeding | Conjunctivitis | |
| Nasal | Bleeding (Epistaxis) | ||
| Oral | Bleeding (gingival) | Mucosal lesions; ulcers, red or white lesions, candidiasis | Odynophagia |
To date there are no reported cases of transmission of Ebola in dental settings. Ebola virus may be transmitted through human secretions, including saliva and, since 1–6% of infected individuals are asymptomatic or mildly symptomatic and the incubation period could last up to 21 days, oral healthcare workers (mainly in the endemic areas) may run the risk of acquiring Ebola if meticulous infection control measures are not always routinely adhered to.7,8
Accurate history, including travel history, contact history, and careful patient examination and the regular careful use of standard/universal infection control measures are probably adequate to minimize any infection risk. A lethal viral infection via needlestick exposure has been reported. The use of disposables wherever possible, and reduction of use of sharps and care in their use, is crucial. Waste disposal regulations must be followed. Transmission through the air has not been documented, nor have there been any reports of transmission through saliva contamination. However, all individuals with detectable serum levels of Ebola RNA also show detectable levels in saliva, thus suggesting a potential transmission.19 Therefore, the fact that it is possibly transmitted through saliva and the incubation period could last up to 21 days implies that oral and dental healthcare workers, especially in the areas where the virus is endemic, such as West Africa and sub-Saharan Africa, may run the risk of acquiring the disease if strict standard infection control measures are not routinely followed. http://www.cdc.gov/oralhealth/infectioncontrol/http://www.cdc.gov/oralhealth/infectioncontrol/guidelines/index.htmhttp://www.cdc.gov/oralhealth/infectioncontrol/guidelines/ppt.htm
In people in whom Ebola is suspected, the CDC guidelines for patients being evaluated for Ebola should be followed (http://www.cdc.gov/vhf/ebola/pdf/checklist-patients-evaluated-us-evd.pdf). The essentials are to:
It would seem prudent, as ADA advises, to defer any elective oral healthcare until at least 21 days have elapsed since Ebola was first suspected as follows:
If dental care is urgent, such as in dental infections and pain, the local health department and patient's physician should be consulted to determine that it is safe to provide such care with standard precautions and physical barriers or, if it is necessary, to follow strictly the specific infection control measures directed to healthcare providers who work with suspected Ebola-infected patients.
The Organization for Safety, Asepsis and Prevention (OSAP) also offers advice, see:
http://www.osap.org/
http://www.oasisdiscussions.ca/2014/11/17/ebo-web/
http://www.osap.org/?page=AboutOSAP
Recommendations on infection control are also released and periodically revised by the CDC and WHO and the two guidelines are similar and include the following measures:
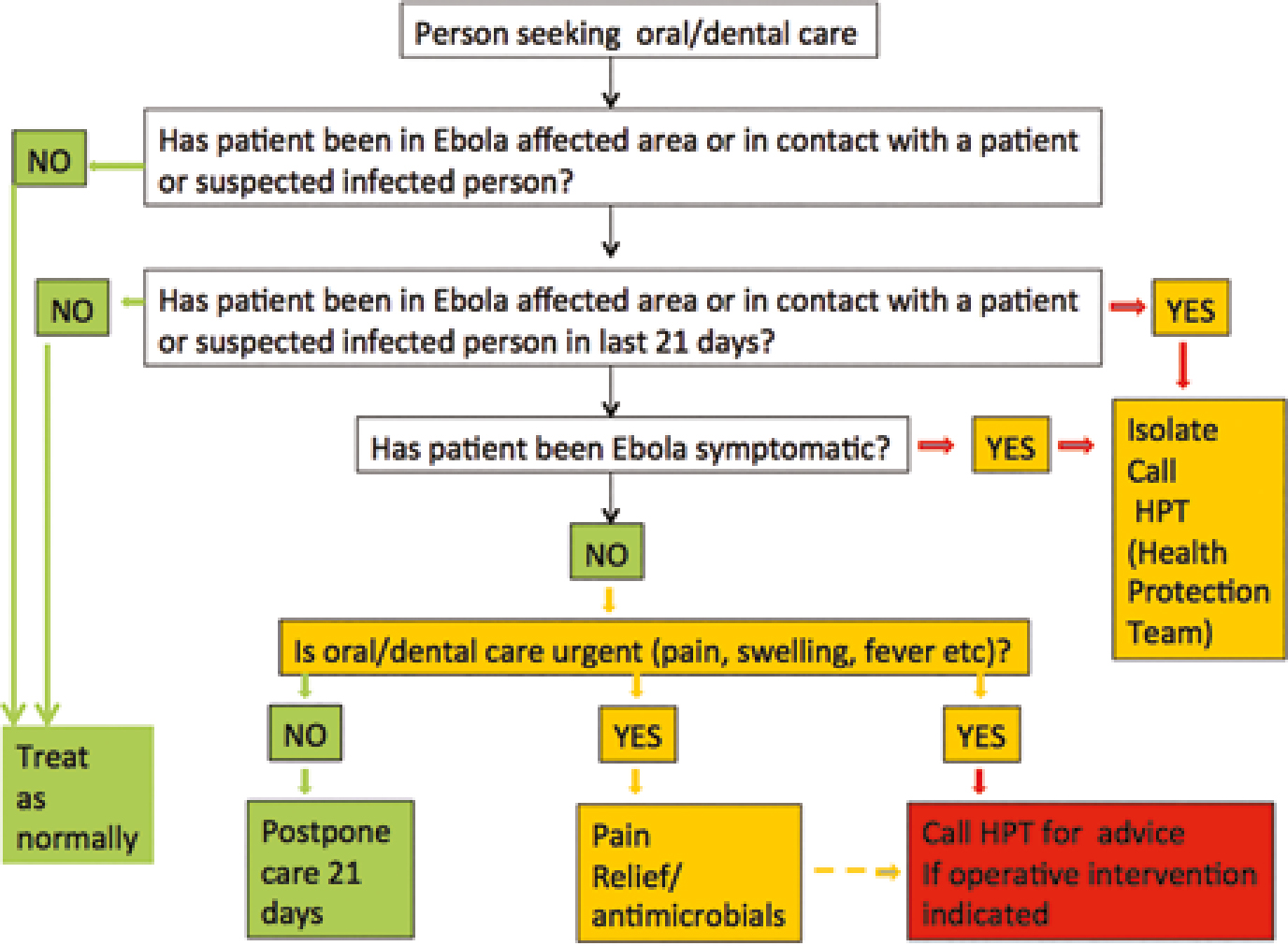
Figure 10 shows an algorithm for handling Ebola in the dental context.
No reliable curative treatment for Ebola is yet available. Patients mostly must rely on supportive therapy. Some infected healthcare workers have been treated with:
It appears that the index case of the current Ebola outbreak started when a child in Meliandou, Guinea was infected by a bat where he played in a hollow tree where bats might roost.
The World Health Organization has formally dispelled rumours of Ebola in Iraq but reports a total of over 20,700 cases and 8,200 deaths in the three most heavily affected West African countries; Guinea, Liberia and Sierra Leone.
Infected or possibly infected healthcare workers from the region have recently been admitted to hospitals in London UK, Berlin, Germany, Nebraska, USA and Lund Sweden. One suffered a needlestick injury.
Trial of the antiviral brincidofovir began on 1 January in Monrovia, Liberia. The Oxford Vaccine Group has started a Phase 1 trial of an Ebola vaccine.
US Food and Drug Administration has approved a study for a device called Hemopurifier to be used in the treatment of Ebola virus disease.
Public Health organizations in UK, such as PHE and the NI administration, have recently published guidance for the dental situation. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/390389/Ebola_guidance_for_dental_care_teams.pdf