Article



Specialist referral may be indicated if the Practitioner feels:
Odontogenic tumours
Odontogenic tumours are rare, are often asymptomatic, and discovered incidentally on imaging (Table 1). They are generally slow-growing and may reach a large size before becoming symptomatic, eg:
| BENIGN |
MALIGNANT |
|---|---|
| Ameloblastoma, solid/multicystic type | Metastasizing (malignant) ameloblastoma |
| Ameloblastoma, extra-osseous/peripheral type | Ameloblastic carcinoma – primary type |
| Ameloblastoma, desmoplastic type | Ameloblastic carcinoma – secondary type (dedifferentiated), intraosseous |
| Ameloblastoma, unicystic type | Ameloblastic carcinoma – secondary type (dedifferentiated), peripheral |
| Squamous odontogenic tumour | Primary intra-osseous squamous cell carcinoma – solid type |
| Calcifying epithelial odontogenic tumour | Primary intra-osseous squamous cell carcinoma derived from keratocystic odontogenic tumour |
| Adenomatoid odontogenic tumour | Primary intra-osseous squamous cell carcinoma derived from odontogenic cysts |
| Keratocystic odontogenic tumour (KCOT) | Clear cell odontogenic carcinoma |
| Odontogenic epithelium with odontogenic ectomesenchyme, with or without hard tissue formation | Ghost cell odontogenic carcinoma |
| Ameloblastic fibroma | Odontogenic sarcomas |
| Ameloblastic fibrodentinoma | Ameloblastoma fibrosarcoma |
| Ameloblastic fibro-odontoma | Ameloblastic fibrodentino and fibro-odonto sarcoma |
| Odontoma (odontome) | |
| Odontoma, complex type | |
| Odontoma, compound type | |
| Odontoameloblastoma | |
| Calcifying cystic odontogenic tumour | |
| Dentinogenic ghost cell tumour | |
| Mesenchyme and/or odontogenic ectomesenchyme with or without odontogenic epithelium | |
| Odontogenic fibroma | |
| Odontogenic myxoma/myxofibroma | |
| Cementoblastoma |
They usually appear as well-defined corticated unilocular or multilocular radiolucencies but, unlike cysts, they are more likely to cause root resorption and buccal and lingual cortical expansion.
The majority of odontogenic tumours are benign. Management is surgical and is dependent upon the type of tumour and varies from enucleation to resection.
Benign odontogenic tumours
Benign odontogenic tumours are 100 times more common than malignant ones: most (>50%) are odontomas, or ameloblastomas (around 10%).
Ameloblastomas are significant since they may recur or metastasize. Composed of ameloblast-like epithelial cells arranged as a peripheral layer around a central area resembling stellate reticulum, two main histological types exist. The follicular type contains discrete islands (follicles) of epithelial cells: the plexiform type consists of anastomosing strands. Ameloblastomas predominate in the posterior mandible, presenting typically in third to fifth decades as a slow-growing, painless, uni-or multi-locular mass (‘soap-bubble’ appearance on imaging) (Figures 1 and 2) usually replacing a tooth and producing more buccolingual expansion and knife edge root resorption than does KCOT (but differentiation is difficult by plain radiography or CT). MRI may then help.
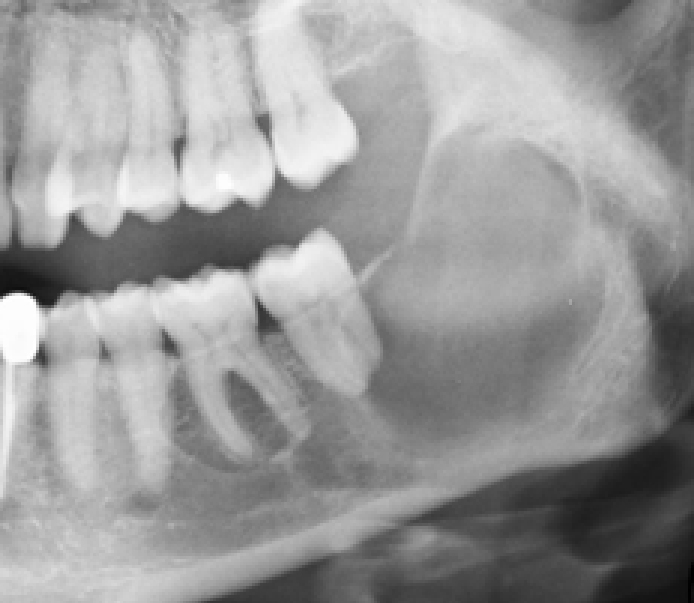
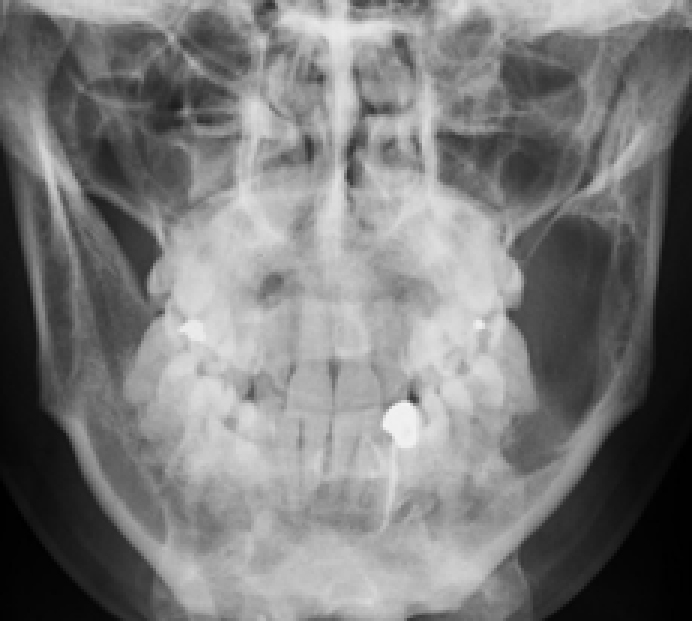
Squamous odontogenic tumour is rare and usually presents as a painless swelling and radiolucency between teeth which become mobile. It may mimic periodontal disease.
Calcifying epithelial odontogenic tumour (CEOT: Pindborg tumour) – rare, benign but aggressive. Three distinct histological features are:
Usually seen in mandibular premolar or molar region associated with the crown of an impacted tooth, CEOT is radiolucent with scattered calcified components.
Adenomatoid Odontogenic Tumour – is the ‘two-thirds tumour’ – most commonly noted in the second and third decades of life and two-thirds of cases:
Sheets and strands of epithelial cells are arranged as convoluted bands and tubular structures, in which ameloblast-like cells are arranged radially around a homogeneous eosinophilic material. It presents as a well-demarcated unilocular radiolucent lesion, often with punctate calcifications. It rarely recurs after excision.
Keratocystic odontogenic tumour (KCOT) has a propensity for destruction and recurrence locally. Radiologically, KCOT usually presents as a well-defined corticated unilocular or multilocular radiolucency which enlarges through cancellous bone, giving rise to late cortical expansion, and for its size the amount of bucco-lingual expansion is small compared to other odontogenic tumours (Figures 3 and 4). The lining has a regular keratinized stratified squamous epithelium, five to eight cell layers thick and without rete pegs. Desquamated keratin is often present within the lumen and the fibrous wall is usually thin. KCOT are often multilocular, well-defined, radiolucent, usually without an associated tooth. The keratin-rich debris shows a characteristic central signal drop on MRI T2-weighted images.
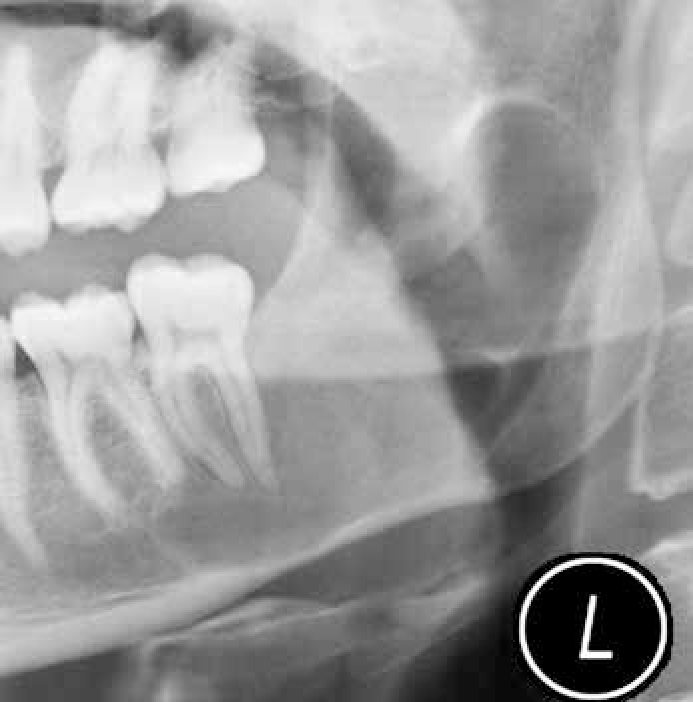
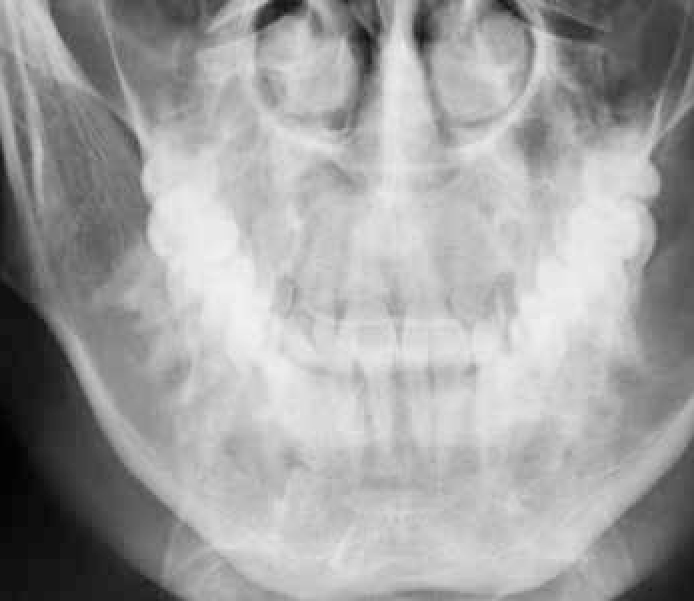
KCOT is associated with chromosome 9 patch gene mutations. Multiple KCOTs in young patients should suggest the basal cell naevus (Gorlin-Goltz) syndrome – an autosomal dominant disorder also with midface hypoplasia, frontal bossing and prognathism, falx cerebri calcification and skeletal anomalies.
Ameloblastic fibroma consists of islands, elongated strands, or terminal buds of ameloblast-like cells and central stellate reticulum cells, surrounded by a cellular hyaline material. It is usually well-defined, pericoronal multiloculated radiolucent and associated with an impacted tooth, often in the posterior mandible.
Odontogenic myxoma is clinically and radiographically indistinguishable from ameloblastoma.
Odontoma (odontome) – a ‘hamartoma’ consisting of dentine and enamel, is often associated with an impacted tooth, classified as follows:
Odontomes typically present during the second decade, are more common in females than males, and often in the mandibular premolar–molar region. Typically, they can behave like teeth: they can grow and tend to erupt, or may displace adjacent teeth. Failure of a tooth to erupt is usually the justifying reason for radiographic exposure which identifies the odontome.
Cementoblastoma is a neoplasm of cementum typically seen in patients under 25 years. Usually fused to a root (typically mandibular premolar or first molar), it is a well-defined radio-opacity with a radiolucent margin. It may cause pain which responds to NSAIDs. Hypercementosis, in contrast, is smoother, less nodular, and has a thin radiolucent margin continuous with the periodontal ligament space.
Malignant odontogenic tumours
These are generally considered as the malignant counterparts of the benign categories (Table 2).
| Tumour type and % of odontogenic tumours | Age in years | Site | Association with unerupted teeth | Resorption of teeth | Other features |
|---|---|---|---|---|---|
| Ameloblastoma 11% | 80%<40 | 90% mandible molar region | 38% (predominantly third molar region) | 39% knife edge | Multilocular; angle of mandible preserved. |
| Ameloblastic fibroma 2% | <20 |
73% mandible molar/premolar | 100% | Rare | Multilocular: less likely to destroy areas of expanded cortex than ameloblastoma. |
| Ameloblastic fibro-odontoma 2% | <20 | Mandible = maxilla 25% anterior jaw | 100% | Rare | Multilocular; very small lesions cause large amount of tooth displacement. |
| Adenomatoid odontogenic tumour 3% | 5–50 Average 16 70% teenage | 75% maxilla 90% in canine incisor region | 74% | Rare | Snowflake opacities evenly arranged throughout lesion. |
| Calcifying epithelial odontogenic tumour 1% | 30–50 peak 40 | Mandible 2x maxilla |
52% rarely impacted Calcification begins | May occur knife edge | Maybe multilocular: lesion tends to extend into body rather than ramus. Less well defined than ameloblastoma. |
| Calcifying cystic odontogenic Tumour 2% | 10–19 | Mandible = maxilla 75% ant to 6 | 20–25% | Occasionally | Hydraulic bone expansion, expanded bone may appear perforated. |
|
Keratocystic odontogenic tumour
|
20–50 | Mandible 3x maxilla | 10% multiple. CT scan if sinus involved. |
||
| Odontogenic fibro/myxoma 3–5% | Mean 25–35 | Mandible 3x maxilla Molar>premolar rare ramus | 5% often congenitally absent tooth | Occasionally | May be multilocular; May destroy angle of mandible |
| Cement-ossifying fibroma 40% of FCOL | 15–50 | 80% mandible premolar and molar Maxilla, zygoma and canine | May occur | Expansion of bone equally in all directions Cortex remains intact Bowing of inferior border parallels tumour mass. | |
| Benign cementoblastoma 9% of FCOL | <25 | 85% mandible |
50% resorption of fusion | Only cemental lesion to be attached to root of involved tooth. | |
| Odontomes 67% | <20 | Compound: Anterior maxilla 62%. Complex: Mandible 1st 2nd molar 70% | 48% | Rare | Compound 2x as common as complex. |
Some useful points with regard to odontogenic tumours are given in Table 3.
|
Normal follicle space
|
>2.5 80% cystic The canine has larger follicle space |
| Radicular cyst or granuloma | Diameter >1.6 cm or Area 200 mm |
| Dentigerous cysts | Lingual expansion rare |
| Keratocystic odontogenic tumour | 10% multiple CT scan if sinus involved. Radioluency in ramus not contacting any teeth most likely keratocyst. Minimal tooth displacement. Perforation of cortex may occur. |
|
Nasopalatine cyst
|
4:1 M:F Diameter >1cm ?cystic Normal well-defined lateral walls only |
| Central Giant Cell Granuloma | F:M 2:1 60% < 20 years of age. Associated with Paget's disease. Rare in ramus; usually anterior to first molar; uneven buccolingual expansion. |
| Aneurysmal bone cyst | 90% < 30 years. Cortex remains intact even when large. Marked buccolingual expansion compared to AP. |
| Central haemangioma | Premature exfoliation of 1º and delayed eruption of 2º teeth. Phleboliths. Vertical expansion important feature |
| Arteriovenous malformation | Cortical thinning without perforation Sunray appearance: Phleboliths |
| Solitary bone cysts | 50% > 3 cm. Few extend into ramus 70% have scalloped upper margin |
|
Ameloblastoma
|
80% < 40 years. 10–15% unilocular |
| Ameloblastic fibroma | Even when small causes buccolingual expansion |
| Ameloblastic odontofibroma | Small lesion large tooth displacement |
| Adenomatoid odontogenic tumour | 74% unerupted tooth. 68% canines, 70% < 20 years, F:M 2:1 |
| Calcifying epithelial odontogenic tumour | Frequently scalloped margin. Variable definition. Expands into body rather than ramus. |
| Calcifying cystic odontogenic tumour | Hydrostatic expansion. Cortical perforation 96% unilocular |
| Odontogenic myxoma | Soap bubble appearance; expansion and perforation May destroy angle |
| Benign cementoblastoma | Attaches to tooth root |
| Cement-ossifying fibroma | Expansion inferior cortex of the mandible |

